Case Report, Arch Clin Pathol Vol: 1 Issue: 1
A Triplet Baby with Progressive Hydrocephalus and Neurodevelopmental Delay
Aye Aye Khine*
Department of Chemical Pathology, National Health Laboratory Services/ School of Medicine, Sefako Makgatho Health Sciences University, Pretoria, South Africa
*Corresponding Author : Aye Aye Khine
Department of Chemical Pathology, National Health Laboratory Services/School of Medicine, Sefako Makgatho Health Sciences University, Pretoria, South Africa
Tel: 012 521 4253/4250
E-mail: ayeaye.khine@smu.ac.za
Received: May 08, 2018 Accepted: June 11, 2018 Published: June 15, 2018
Citation: Khine AA (2018) A Triplet Baby with Progressive Hydrocephalus and Neurodevelopmental Delay. Arch Clin Pathol J 1:1.
Abstract
A 4-month old baby girl, one of the Triplets born premature at 34 weeks of gestation was admitted at one of the South Africa’s referral hospital due to severe vomiting, progressive hydrocephalus with neuro-developmental delay. The other two siblings demised shortly after birth. The mother was known HIV positive on the HAART and the baby tested negative using HIV DNA PCR. Three consecutive samples of cerebrospinal fluid (CSF) from the shunt were sent to the laboratory for analysis. The baby did not have any fever, neck stiffness or seizures.
Four consecutive CSF samples showed clear appearance, decreased glucose levels with increase in the adenosine deaminase enzyme levels (ADA). CSF protein was normal and microscopy showed absence of cells. Due to the high ADA activity, the CSF microscopy for acid fast bacilli and culture for general bacteria and Mycobacterium Tuberculosis (MTB) were performed and both were negative. CT scan brain showed large multiple cysts with enlarged lateral ventricles. MRI was not done because it was not available at that time.
In TORCH screening, apart from the CMV (IgG), the rest were negative. The former was very high in titre in the mother’s blood, but negative in the baby. Both mother and baby tested negative for CMV IgM. In addition, three consecutive urine samples from the baby were collected for CMV detection by the polymerase chain reaction (PCR) and they were all negative. Absence of maternal CMV IgG in the baby at this age was unusual and repeated twice to confirm the negative result. CSF PCR for both MTB and CMV were also negative.
Differential diagnoses were periventricular leukomalacia (PVL) either from CMV encephalopathy or pre/perinatal stroke (hypoxic ischemic brain injury), as in both conditions the multiple periventricular cysts formation is a shared characteristics. On the basis of negative CMV PCR test, antiviral therapy was not considered. It is, however, to note that positive rate of CMV PCR in neonatal period is very low despite presence of infection.
Conclusion: Perinatal or intrauterine CMV infection is very difficult to prove. It is recommended that evaluation of such cases require MRI scanning of central nervous system which can reveal calcifications in the light of difficulties in finding the viral genome by nucleic acid testing. Patient would have benefited from the anti-viral therapy from alleviating further brain damage.
Keywords: CMV infection; Hydrocephalus; Neurodevelopmental delay; Adenosine deaminase enzyme
Case Description
A 4-month old female infant was prematurely born from a vaginal Triplet delivery and referred to the hospital with a progressive hydrocephalus, vomiting and neuro-developmental delay. Her siblings demised shortly after birth. She was HIV PCR negative although her mother was positive and had been on treatment (Table 1).
| Unit | admission | 2nd week | 3rd week | 4th week | Ref range | |
|---|---|---|---|---|---|---|
| Appearance | clear | clear | clear | clear | ||
| CSF-Chloride | Mmol/l | 103 | 117 | 115 | 111 | 118-132 |
| CSF-Glucose | Mmol/l | 0.5 | 2.2 | 2.0 | 1.5 | 2.7-4.4 ( or >60% of plasma glucose) |
| Plasma glucose | Mmol/l | 3.5 | 4.2 | 4.5 | 3.2 | |
| CSF-T Protein | g/l | 0.34 | 0.23 | 0.27 | 0.26 | 0.15-0.40 |
| CSF-ADA | IU/L | 43 | 34 | 37 | 23 | 0-6 |
Table 1: CSF Biochemistry and Adenosine Deaminase Levels during Admission.
Laboratory Investigations
Cerebro-jugular shunt was inserted to release the intracranial pressure. Four consecutive CSF samples were taken from the shunt for analysis. They were all clear visibly and biochemistry consistently showed normal protein, decreased glucose levels with increase in the adenosine deaminase enzyme levels (ADA). Microscopy showed absence of cells. Due to the high ADA activity, the CSF microscopy for acid fast bacilli and culture for general bacteria and Mycobacterium Tuberculosis (MTB) were performed and both were negative.
TORCH (Toxoplasmosis, Rubella, Syphilis, Cytomegalovirus, and Herpes) serology screen for both mother and baby were done to exclude perinatal infections and apart from the Cytomegalo virus infection (CMV), the others were negative. The CMV Immunoglobulin G (IgG) was very high in titre in the mother’s blood, but negative in the baby. Both mother and baby tested negative for CMV IgM. The mother has a high CMV IgG avidity index during the antenatal period of this pregnancy. In addition, three consecutive urine and CSF samples from the baby were collected during admission for CMV detection by the polymerase chain reaction (PCR) and they were negative.
Imaging Study
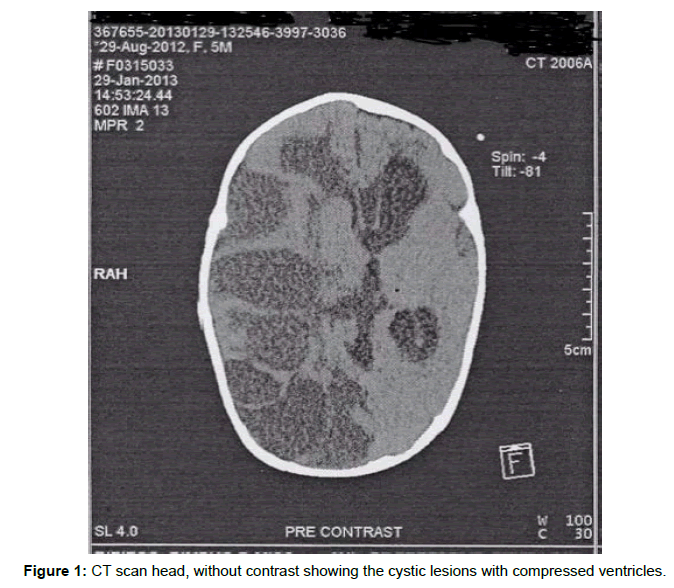
Based on the radiological report, the right cerebral hemisphere showed multiple cystic areas of varying sizes with most of them being extra-axial. There was prominent architectural distortion in the right cerebral hemisphere with distinctly low density of the white matter. There was also severe cortical thinning and reduced grey to white matter differentiation within the parietal and occipital region. The right lateral ventricle was compressed whilst the left was distended with a midline shift toward the left measuring approximately 8 mm. No calcifications were seen (Figure 1).
Discussion
Consultations with Paediatricians and Neurosurgeons led to two differential diagnoses: 1) periventricular leukomalacia (PVL) as a result of possible peri-natal hypoxic ischemic brain injury, and 2) CMV encephalopathy as they share characteristics of the multiple peri-ventricular cysts formation [1,2]. The PVL can be commonly found in premature neonates especially in Triplets [3]. PVL has also been reported as the most common complication in neonates with CMV infection [4].
Brain scan findings such as absence of calcifications along the cyst margins and around the ventricles together with the negative PCR put the CMV encephalopathy as a second choice. However, it was noted that magnetic resonance imaging would be a better resolution to reveal the fine areas of calcifications [5]. This hospital is poorly resourced and the patients have to wait for a long time for a MRI.
On the other hand, negative PCR tests in the CSF and in three consecutive urine samples could not totally exclude this infection since the screening was rather late (not immediately after patient started presentation) and also because the virus only sheds intermittently in the urine [6]. Mother’s CMV IgG high avidity also suggested that the infection was rather in the past than recent in this pregnancy [7]. This was not in favour of intrauterine CMV infection.
In the CSF examinations, high Levels of ADA has been reported in a Patient with Herpetic Encephalitis who’s Initial PCR for Herpes was negative but the CSF mononuclear cell count was 11 cells/ml [8]. Persistence of increased ADA activity in the CSF is a dilemma in this case especially due to lack of mononuclear cells in the same specimens. Typical CSF findings in most cases of cerebral CMV infection are an elevated protein level and mononuclear leucocytosis. CMV is rarely detected by PCR in the CSF, however positive finding helps confirm the diagnosis. Electrolyte disturbances (eg., hyponatremia) consistent with adrenal insufficiency may be observed although in this baby, electrolytes and renal functions were normal [9].
After discharge, the infant continued with a progressive neurodevelopmental delay although the head circumference had not grown since the shunt was removed. Hearing tests were performed during follow up visits and showed stage 2 impairment. Serological test for CMV and urine CMV PCR were repeated after 3 month and IgG became positive. The negative serological tests at the time of admission could be due to low level antibodies undetectable with the test kits used by the laboratory. The urine CMV PCR only became positive after 6 month of discharge from the hospital, thus the diagnosis was made as CMV encephalopathy although the time of infection could not be confirmed.
However, severity of cerebral involvement explored the question of associated hypoxic brain injury. Although the mother did not recall any birth trauma or difficulty in labour, the fact that her Triplet pregnancy was undiagnosed until after the first baby was born could have put the Triplets particularly at risk for intra-uterine hypoxic brain injury and the stillbirth of the other 2 babies. Elovaara et al. [10] reported a lowered CSF leukocyte count in 72 HIV infected young patients with predominantly brain atrophy which may be used as a guide to lack of cells in the CSF in this case although it is a different viral infection.
Final Diagnosis
CMV encephalopathy with intra-uterine hypoxic brain injury.
Conclusion
Diagnosis of CMV infection in neonates poses particular challenges due to sensitivity of serology tests and low positive rates in the viral isolation and PCR tests. High index of suspicion in the background of patient’s history, timeous reliable imaging and repeat serology and virology testing are required. If there are other confounding events such as brain hypoxia, the diagnostic dilemma can delay the treatment of CMV infection.
Teaching Points
In multiple pregnancy, neonates are prone to perinatal infections such as Cytomegalo virus as well as hypoxic brain injury and they may present with progressive hydrocephalus
1. MRI is better than CT scan to confirm the presence of calcifications at the cyst margins and around the ventricles does favour the diagnosis of CMV whilst severity of periventricular leukomalacia points more towards intrauterine hypoxic brain injury. However, they can co-exist.
2. Low levels of CMV IgM in the neonate could lead to false negative results.
3. Maternal CMV IgG screening should include avidity index testing to discern recent infection from past infection.
4. If CSF is not accessible, urine can be used for CMV PCR and can be repeated if the initial sample is negative and clinical suspicion is high. Early confirmation of CMV infection is important as anti-viral therapy can prevent permanent brain damage.
Acknowledgements
1. Dr T Kyaw from Virology, NHLS/SMU, South Africa and consultants from the Neurosurgery department, Dr George Mukhari Academic Hospital for their contributions in the clinical consultations during working up the diagnosis of this case.
2. Mother of the baby for providing informed consent in publishing this work.
References
- Govaert P (2009) Prenatal stroke. Semin Fetal Neonat 14: 250–266.
- Manara R, Balao L, Baracchini C, Drigo P, D’Elia R, et al. (2011) Brain magnetic resonance findings in symptomatic congenital cytomegalovirus infection. Pediatr Radiol 41: 962–970.
- Iliodromiti Z, Zygouris D, Karagianni P, Belitsos P, Daniilidis A, et al. (2012) Brain injury in preterm infants, Intechopen Limited, USA, 73–86.
- Boesch C, Issakainen J, Kewitz G, Kikinis R, Martin E, et al. (1989) Magnetic resonance imaging of the brain in congenital cytomegalovirus infection. Pediatr Radiol 19: 91–93.
- van der Knaap MS, Vermeulen G, Barkhof F, Hart AM, Loeber JG, et al. (2004) Pattern of white matter abnormalities at MR imaging: use of polymerase chain reaction testing of Guthrie cards to link pattern with congenital cytomegalovirus infection. Radiology 230: 529–536.
- Haginoya K, Ohura T, Kon K, Yagi T, Sawaishi Y, et al. (2002) Abnormal white matter lesions with sensorineural hearing loss caused by congenital cytomegalovirus infection: Retrospective diagnosis by PCR using Guthrie cards. Brain Dev 24: 710–714.
- Harry E, Lapé-Nixon M (2014) Role of Cytomegalovirus (CMV) IgG avidity testing in diagnosing primary CMV infection during pregnancy. Clin Vaccine Immunol 21: 1377–1384.
- Lopes-Gomez M, Lopes-Ruz M, Jimenez-Alonso J (2003) High levels of adenosine deaminase in a patient with herpetic encephalitis and initial negative PCR results. Clin Infect Dis 37: 147–148.
- Uppal G (2015) Cytomegalo virus encephalitis in HIV infected neonates. Mayo Clinic Proceedings, New York, USA.
- Elovaara I, Poutiainen E, Raininko R, Valanne L, Virta A, et al. (1990) Mild brain atrophy in early HIV infection: the lack of association with cognitive deficits and HIV-specific intrathecal immune response. J Neurol Sci Nov 99: 121-136.