Research Article, J Appl Bioinforma Comput Biol Vol: 7 Issue: 4
Anti-Angiogenic Potential of Secondary Metabolites against Tyrosine Kinase Domain of Vascular Endothelial Growth Factor Receptor-1: An in silico Approach
Upendra N. Dwivedi*1,2, Aleena Asif1, Sameeksha Tiwari1,2, Om Prakash2, Veda P. Pandey2 and Kusum Yadav2
1Institute for Development of Advanced Computing, ONGC Centre for Advanced Studies, University of Lucknow, Uttar Pradesh, India
2Bioinformatics Infrastructure Facility, Centre of Excellence in Bioinformatics, Department of Biochemistry, University of Lucknow, Lucknow, India
*Corresponding Author: Upendra N Dwivedi
Professor of Biochemistry & Director, Institute for Development of Advanced Computing, ONGC Centre for Advanced Studies, University of Lucknow, Lucknow-226007, Uttar Pradesh, India
Tel: (+91) 522-2740132
E-mail: upendradwivedi@hotmail.com
Received: October 25, 2018 Accepted: December 24, 2018 Published: December 31, 2018
Citation: Dwivedi UN, Asif A, Tiwari S, Prakash O, Pandey VP, et al. (2018) Anti-Angiogenic Potential of Secondary Metabolites against Tyrosine Kinase Domain of Vascular Endothelial Growth Factor Receptor-1: An in silico Approach. J Appl Bioinforma Comput Biol 7:4.
Abstract
Angiogenesis, i.e. formation of new blood vessels, is a key hallmark of tumor growth and progression. The tyrosine kinase (TK) domain of vascular endothelial growth factor receptor (TK-VEGFR-1) is reported as the key intracellular domain responsible for the downstream signalling leading to angiogenesis. Therefore, targeting TK domain of VEGFR-1 and blocking downstream signalling is considered as a promising approach in cancer therapy. Furthermore, in view of severe side effects exhibited by present day synthetic drugs, directed against TK domain of VEGFR-1, there is a worldwide effort to identify safer alternatives drugs, especially coming from natural sources. Keeping this perspective in mind, in the present paper, we have evaluated the anti-angiogenic potential of ADMET screened 18 alkaloids, 26 flavonoids and 9 terpenoids against TK domain of VEGFR-1 through molecular docking approach. Results of the analyses revealed that the alkaloid liriodenine (-7.10 Kcal/Mol), flavonoid glabridin (-9.85 Kcal/mol) and terpenoid sarsasapogenin (-9.58 Kcal/mol) were found to be the best among respective classes. However, across the three classes, flavonoid glabridin was found to be the most potent inhibitor. An assessment of anti-angiogenic potential of the flavonoid glabridin with that of FDA approved drug regorafenib revealed comparable results. Results of docking were further validated using molecular dynamics simulation (MDS) analyses. Thus, the present study makes a foundation for further investigations based on the experimental data (wet laboratory data) for therapeutic application of screened secondary metabolites in general and glabridin and sarsasapogenin in particular
Keywords: Angiogenesis; Docking; Glabridin; Liriodenine; Molecular dynamics simulation; Regorafenib; Sarsasapogenin; Secondary metabolites; TK domain of VEGFR-1
Keywords
Angiogenesis; Docking; Glabridin; Liriodenine; Molecular dynamics simulation; Regorafenib; Sarsasapogenin; Secondary metabolites; TK domain of VEGFR-1
Introduction
Angiogenesis, i.e. the formation of new blood vessels, key feature for growth and development of solid tumors, comprises of series of signalling cascades [1]. One of the key growth factor which is primarily responsible for angiogenesis is vascular endothelial growth factor-A (VEGF-A) [2,3]. VEGF-A has been reported to bind on its receptor namely, vascular endothelial growth factor receptor (VEGFR-1) (also known as fms-like tyrosine kinase (Flt-1) [4,5]. Human VEGFR-1, made up of 1,338 amino acids, has four characteristics domains namely, namely, the extracellular (immunoglobin-like) domain, transmembrane (TM), domain, intracellular kinase (TK) domain, followed by carboxyl terminal (CTD) domain [6]. The binding of a ligand to the extracellular domain of VEGFR-1 leads to dimerization and subsequent activation of tyrosine kinase (TK) domain [7,8]. The activated TK domain further mediates downstream signalling leading to the exposure of ATP binding site of the intracellular TK domain, kinase activation and subsequent auto/transphosphorylation of tyrosine residues on the receptor [9]. Further downstream signal transduction includes the activation of Ras–extracellular regulated kinase (ERK), mitogen activated (MAP) kinase pathway, the phosphoinositide 3-kinase (PI 3-kinase)–Akt and the JAK/STAT pathway subsequently leading to modulation of various subsets of genes. These activation cascades ultimately result in the establishment of biological responses such as cell proliferation, migration and arrangement in three dimensions to form vascular vessels [10].
TK domain of VEGFR-1 has been exploited for therapeutic applications based on the observation that drugs binding at TK domain leads to inhibition of further downstream signalling [11]. Therefore, TK domain of VEGFR-1 has emerged as the promising target for the anti-angiogenesis cancer therapeutics [12,13]. Thus, monoclonal antibodies (VEGF mediated), antibodies conjugates, small molecules as well as plant derived phytochemicals have been tested for their potential for inhibition of the signalling cascades [14,15]. Bevacizumab, (a humanised monoclonal antibody), approved antiangiogenic drug for patients with metastatic colorectal cancer (CRC), non-small cell lung cancer and metastatic breast cancer is being used in combination with chemotherapy [16,17]. Likewise, FDA approved drugs such as sorafenib and sunitinib, are being used in patients with advanced renal cell carcinoma (RCC) and hepatocellular carcinoma (HCC) [18,19]. Axitinib, a multi-kinase inhibitor has been shown to inhibit angiogenesis, vascular permeability and blood flow [20]. Similarly, pazopanib, another FDA approved drug, a potent inhibitor of VEGFR-1, (also VEGFR-2 and VEGFR-3), is being used for the treatment of RCC and ovarian cancer [21].
Although these drugs, directed against VEGFR-1, have displayed delayed tumor progression, leading to overall cell survival compared with standard chemotherapy, however, their long term use has been reported to cause toxicities such as severe bleeding, disturbed wound healing, gastro-intestinal perforation, hypertension, and fatigue [22]. Therefore, there is an urgent need to look for safer drugs with lesser toxicity in their long term use. In this direction, drugs derived from natural sources such as plants have attracted the attention of people worldwide. Thus, phytochemicals such as alkaloids (nonprotein nitrogen-containing compounds), flavonoids (polyphenolic compounds) and terpenoids (polymeric isoprene derivatives) have been strategically used in cancer therapeutics as they have little or no side effects in their long term use. Furthermore, plant derived phytochemicals offer additional advantages of being cheap, stable and easy to procure. Therefore, these aforesaid phytochemicals can offer excellent leads for drug discovery.
A number of alkaloids have been reported to possess potent antineoplastic properties. Thus, alkaloids namely, taxol and camptothecin have been used for the treatment of metastatic ovarian cancer [23]. Among the different alkaloids exhibiting anti-cancerous properties, pyridocarbazoles (ellipticines) have also been reported for their property to suppress p53 activity, one of the hallmarks of cancer [24,25].
Similarly, flavonoids and their synthetic analogs have been extensively investigated in the treatment of ovarian, breast, cervical, pancreatic, and prostate cancers. Flavonoids such as curcumin, possesses anti-inflammatory, anti-oxidant, anti-proliferative, antiangiogenic, and antineoplastic properties [26]. It is also reported that curcumin in combination with drug tamoxifen has been used in the treatment of melanoma [27]. Furthermore, curcumin has also been reported to block the expression of EGFR (epidermal growth factor receptor), VEGFR-1, VEGFR-2 and VEGFR-3, and the kinase activity of proto-oncogenes such as Src, which are responsible for the induction of angiogenic genes as well as endothelial cell migration and proliferation [28]. Also, flavonoids like genistein and luteolin have been reported to have inhibitory effect on cancer cells [29,30]. The flavonoid apigenin, has also been reported to suppress the expression of HIF-1 and VEGF via PI3K/AKT/p70S6K1 and HDM2/ p53 signalling path-ways, blocking the signalling pathways in cancer cells under both normal and hypoxic conditions; thus, preventing formation of new blood vessels via CAM [31]. Terpenoids like D-limonene also possess anti carcinogenic properties and hence, quiet used in cancer preventive therapy against mammary, liver, skin, lung, colon, stomach, prostate, and pancreatic cancer [32,33]. Likewise, betulinic acid, a triterpenoid has also been reported to exhibit anti carcinogenic activity against several human tumor cells, including melanoma and glioma [34]. In addition, salvicine has been reported to possess significant anti-tumor activity, against malignant tumors, especially solid tumors such as lung and gastric cancers [35]. Withaferin A, a key steroidal lactone, has been reported to exert potent anti-angiogenic activity [36].
In the present paper, selected plant secondary metabolites belonging to alkaloids, flavonoids and terpenoids (ADMET screened 18 alkaloids, 26 flavonoids and 9 terpenoids) and ten FDA approved known inhibitors (i.e. drugs) have been investigated and compared for their inhibitory potential against TK domain of VEGFR-1, a crucial therapeutic angiogenic cancer target, using molecular docking approach [37-39]. The result of the best docked secondary metabolite was finally validated and compared with the FDA approved drug regorafenib (taken as control) using molecular dynamics (MD) simulation analyses. The finding of the present investigation is a step towards use of safer and efficient therapeutics derived out of natural compounds.
Materials and Methods
Structure preparation of TK domain of VEGFR-1
The 3D crystal structure of intracellular tyrosine kinase domain (TK domain) of VEGFR-1 receptor in complex with N-(4- Chlorophenyl)-2-((pyridin-4-ylmethyl) amino) benzamide (PDB ID: 3HNG) was retrieved from Protein Data Bank (PDB). The 3D structure of TK domain of VEGFR-1 was refined by removing the bound ligand i.e.N-(4-Chlorophenyl)-2-((pyridin-4-ylmethyl) amino) benzamide with the help of molecular viewer UCSF Chimera.
Preparation of ligand structure
The molecular files of FDA approved drugs namely, axitinib, lenalidomide, lenvatinib, motesanib, regorafenib, sunitinib, thalidomide, vandetanib and vatalanib as well as selected plant derived secondary metabolites belonging to alkaloids, flavonoids and terpenoids were retrieved from NCBI PubChem database. Among selected plant derived secondary metabolites there were 18 alkaloids (anonaine, aristolactam, canthin-6-one N-oxide, coptisine, corydine, crebanine, dehydrocorydaline, dicentrine, eleuthrin, glaucine, liriodenine, lunacridine, lycorine, noscapine, oliveroline, oxostephanine, piperine, protopine), 26 flavonoids (acacetin, apigenin, baicalein, cajanol, chrysin, curcumin, deguelin, galangin, genkwanin, glabridin, helichrysetin, lethedocin, licochalcone, morindone, mucronulatol, nobiletin, pongavilleanine, pseudobaptigenin, rotenolone, rotenone, sinensetin, tangeretin, tephrosin, velutin, wogonin, zapotin) and 9 terpenoids (carnosol, cynaropicrin, limonene, mansononeE, menthol, salvicine, sarsasapogenin, sclareol, tanshinone) which were previously pharmacokinetic (ADMET) screened [37-39]. All the mol files of these ligands (drugs and secondary metabolites) were converted to Protein Data Bank (PDB) format using Open Babel [40].
Molecular docking
Docking analyses of the selected drugs and secondary metabolites were performed using Autodock 4.2 software, a tool based on Lamarckian Genetic Algorithm (LGA), for estimating binding energy and inhibitory constant [41]. Before starting the molecular docking, the structure of the target protein, TK domain of VEGFR-1, was prepared by adding polar hydrogen atoms and Gasteiger charges were calculated for each atom of the target protein. Likewise, the ligand structures were also prepared as per default settings. After preparation of target and ligand files the active site for the interaction of ligand with protein was defined. For this, AutoGrid program was used to set grid maps 80 × 80 × 80 A°(x, y and z) for ligands (i.e. drugs and secondary metabolites) with 0.375 A° spacing. The docked model in the lowest energy cluster was considered for all further interaction studies. The binding energy and inhibition constant (Ki) are expressed as kcal/mol and micromolar (μM) units, respectively.
MD Simulation
Interactions of the best identified secondary metabolite flavonoid glabridin along with the best docked drug regorafenib, at the active site of TK domain of VEGFR-1 were investigated through 25 ns molecular dynamics (MD) simulation analyses using GROMACS 4.5.5 package with CHARMm27 force field. The docking poses of TK domain of VEGFR-1 with best docked flavonoid and drug regorafenib were prepared for MD simulation through mild minimization and salvation within a water-filled 3D cube of 1 A° spacing. System was neutralized and further minimized. The complex structure was heated to 300 K and equilibrated for 100 psin NVT ensemble and another 100 ps in NPT ensemble. After heating and equilibration, the complex structure of TK domain of VEGFR-1 with its ligands were subjected to production run of 25 ns in NPT ensemble. PRODRG web server was used to generate topologies and co-ordinations of ligands [42].
Active site mapping of best docked complexes of TK domain of VEGFR-1 with secondary metabolites and drugs
Regiospecificity of interactions of best docked secondary metabolites (belonging each of alkaloids, flavonoids and terpenoids) and the best drug with that of the TK domain of VEGFR-1 were visualised using a molecular viewer Chimera, developed and maintained by the University of California, San Francisco, CA, USA [43].
Results
Molecular docking analyses of drugs with TK domain of VEGFR-1
The TK domain of VEGFR-1 was docked with ten FDA approved drugs. Results of docking analyses are presented in Table 1. It is noteworthy that on the basis of binding energy and Ki, regorafenib (Ki=5.02 ×10-4 μM) was found to be most potent while that of pegaptanib (Ki=3800 μM) was the weakest.
| S.No | Name of the drug | Binding energy (Kcal/mol) | Ki (µM) | Interacting residues at the active site | Hydrogen donor atom | Hydrogen acceptor atom | Distance(A°) |
|---|---|---|---|---|---|---|---|
| 1 | Axitinib | -7.41 | 3.73 | Ile 1019, His 1020, Cys 1039, Asp 1040, Phe 1041, Gly 1042 | Axitinib:H39 | ASP 1040:OD1 | 1.69805 |
| 2 | Lenalidomide | -6.9 | 8.82 | Ile 1019,His 1020, Arg 1021, Cys 1039, Asp 1040, Phe 1041 | CYS1018:SG ARG1021:NH2 Lenalidomide :H27 Lenalidomide:H31Lenalidomide:H31 | Lenalidomide:OD1 Lenalidomide:O3 ILE 1019:O, GLU 878:OE2 ASP 1040:O | 3.50775 2.78229 2.03957 3.02604 2.23548 |
| 3 | Lenvatinib | -9.14 | 0.201 | Ile 1038, Cys 1039, Asp 1040 | Lenvatinib:H49 | ASP 1040:OD1 | 1.89093 |
| 4 | Motesanib | -6.61 | 14.25 | Ile 1019,His 1020, Cys 1039, Asp 1040 | CYS 1018:SG, Motesanib:H31Motesanib:H31 | Motesanib:N6, HIS 1020:OASP 1040:OD1 | 3.01791 2.6665 2.31529 |
| 5 | Pegaptinib | -3.3 | 3800 | Cys 1039, Asp 1040, Phe 1041, Gly 1042, Leu 1043 | ASP1040:N Pegaptinib:H56 Pegaptinib:H70 | UNK0:O8 ASP1040:OD1 ASP1040:O | 3.15359 1.72805 1.941 |
| 6 | Regorafenib | -9.96 | 5.02 ×10-4 | Ala 859, Val 860, Val 861,Cys 1018, Ile 1019, His 1020, Arg 1021, Ile 1038, Cys 1039, Asp 1040, Phe 1041 | CYS 1018:SG ARG1021:NE ARG1021:NHE Regorafenib:H45 | Regorafenib:O6 Regorafenib:O8 Regorafenib:N11 ASP1040:OD1 | 2.68932 2.75537 3.13925 2.0852 |
| 7 | Sunitinib | -8 | 1.38 | Tyr 911, Cys912, Ile 1038, Cys 1039, Asp 1040, Phe 1041 | CYS 912:N ASP1040:N Sunitinib:H34 | Sunitinib:F1 Sunitinib:O3 ILE 1038:O | 2.71848 2.97853 2.76497 |
| 8 | Thalidomide | -6.95 | 8.11 | Ile 1019, His 1020, Arg 1021, Cys 1039, Asp 1040 | ARG1021:NH2, Thalidomide :H25 Thalidomide:H25 | Thalidomide:O4 ILE 1019:O HIS 1020:O | 3.15659 2.70627 2.09873 |
| 9 | Vandetanib | -9.56 | 0.098 | Cys 1018, Ile 1019, His 1020, Cys 1039, Asp 1040 | - | - | - |
| 10 | Vatalanib | -7.55 | 2.93 | Glu 878, Thr 877, Ile 1038, Cys 1039, Asp 1040,Phe 1041 | - | - | - |
Table 1: Result of docking of FDA approved drugs with TK-VEGFR-1 depicting binding energy, Ki, interacting residues, hydrogen bond donor and acceptor atoms involved in bonding and their distance at the active site of TK domain of VEGFR-1.
Active site mapping of docked complex of TK domain of VEGFR-1 with drugs
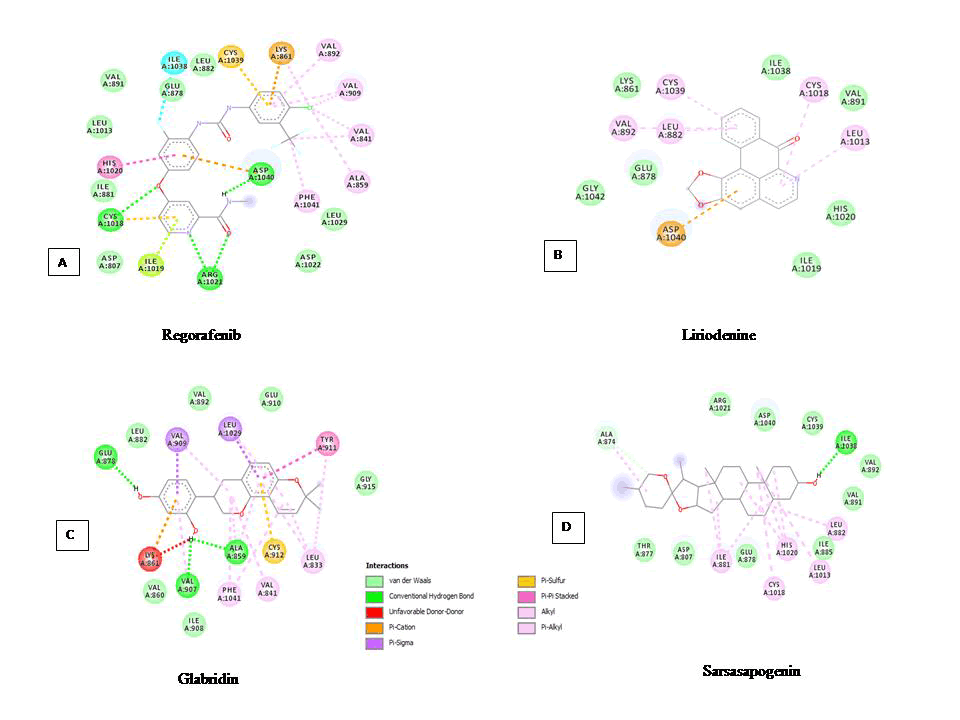
The interaction of drugs with the TK domain of VEGFR-1, at the active site within 5 A°, was analysed and compared (Table 1). From the data, it is evident that all the drugs are binding at more or less same binding pocket within the active site of the TK domain of VEGFR-1 as evident by the fact that majority of the interacting residues are common. From the (Figure 1A) it can be concluded, that the most potent drug regorafenib, interacts with total eleven residues, namely, Ala859, Val860, Val861, Cys1018, Ile1019, His1020, Arg1021, Ile1038, Cys1039, Asp1040, Phe1041. Out of these residues, residues namely, Cys1018, Arg1021 and Asp1040 are involved in four H-bond interactions. It is also noteworthy that the residues namely, Cys1039 and Asp1040 are common for all ten drugs. Similarly, residues like ILE1019 and HIS1020 are common for the five drugs such as axitinib, lenalidomide, motesanib, regorafenib and thalidomide. Drugs like regorafenib and vandetanib have two common residues such as ALA859 and VAL860. In drugs, vandetanib and vatalanib no H-bond interaction was found.
Molecular docking analyses of selected alkaloids, flavonoids and terpenoids with TK domain of VEGFR-1
The selected 18 alkaloids, 26 flavonoids and 9 terpenoids, fulfilling the ADMET criteria, were analysed for their inhibitory potential against the TK domain of VEGFR-1 by molecular docking approach. Data depicting binding energy and Ki, are presented in Table 2. The data revealed that out of 18 alkaloids, liriodenine (Ki=6.30 μM) was found to be the most potent inhibitor of TK domain of VEGFR-1 while that of oliveroline (Ki=86.35 μM) was found to be weakest. Similarly, in case of flavonoids, out of 26 flavonoids, glabridin (Ki=0.060 μM) was found to be the most potent inhibitor while that of tangeretin (Ki=633.83 μM) was found to be weakest. Furthermore, out of 9 terpenoids, sarsasapogenin (Ki=0.095 μM) was found to be the most potent inhibitor while that of menthol (Ki=121.39 μM) was weakest.
| S.No | Secondary metabolites | Binding energy (Kcal/mol) |
Ki value (µM) |
S.No | Secondary metabolites | Binding energy (Kcal/mol) |
Ki value (µM) |
|---|---|---|---|---|---|---|---|
| Alkaloids | 27 | Genkwanin | -7.67 | 2.83 | |||
| 1 | Anonaine | -7.05 | 6.82 | 28 | Glabridin | -9.85 | 0.060 |
| 2 | Aristolactam | -6.87 | 9.23 | 29 | Helichrysetin | -7.32 | 4.29 |
| 3 | Canthin-6-one N-oxide | -6.21 | 27.96 | 30 | Lethedocin | -5.64 | 73.51 |
| 4 | Coptisine | -5.92 | 46.02 | 31 | Licochalcone | -6.90 | 8.73 |
| 5 | Corydine | -6.01 | 39.23 | 32 | Morindone | -7.21 | 5.13 |
| 6 | Crebanine | -6.56 | 15.48 | 33 | Mucronulatol | -7.39 | 3.84 |
| 7 | Dehydrocorydaline | -6.17 | 29.88 | 34 | Nobiletin | -7.83 | 1.82 |
| 8 | Dicentrine | -6.65 | 13.36 | 35 | Pongavilleanine | -7.29 | 4.52 |
| 9 | Eleuthrin | -6.89 | 8.97 | 36 | Pseudobaptigenin | -7.10 | 6.21 |
| 10 | Glaucine | -6.36 | 21.60 | 37 | Rotenolone | -7.64 | 2.52 |
| 11 | Liriodenine | -7.10 | 6.30 | 38 | Rotenone | -7.43 | 3.60 |
| 12 | Lunacridine | -6.19 | 29.07 | 39 | Sinensetin | -7.90 | 1.62 |
| 13 | Lycorine | -6.87 | 9.20 | 40 | Tangeretin | -4.36 | 633.83 |
| 14 | Noscapine | -5.69 | 66.93 | 41 | Tephrosin | -8.16 | 1.04 |
| 15 | Oliveroline | -5.54 | 86.35 | 42 | Velutin | -7.11 | 6.18 |
| 16 | Oxostephanine | -6.89 | 8.89 | 43 | Wogonin | -6.50 | 17.17 |
| 17 | Piperine | -6.02 | 38.35 | 44 | Zapotin | -5.83 | 53.60 |
| 18 | Protopine | -5.91 | 46.65 | Terpenoids | |||
| Flavonoids | 45 | Carnosol | -7.96 | 1.46 | |||
| 19 | Acacetin | -7.11 | 6.13 | 46 | Cynaropicrin | -8.33 | 0.787 |
| 20 | Apigenin | -7.59 | 2.75 | 47 | Limonene | -5.44 | 102.67 |
| 21 | Baicalein | -6.31 | 23.73 | 48 | MansononeE | -6.43 | 19.23 |
| 22 | Cajanol | -6.79 | 10.59 | 49 | Menthol | -5.34 | 121.39 |
| 23 | Chrysin | -6.83 | 9.87 | 50 | Salvicine | -6.87 | 9.20 |
| 24 | Curcumin | -6.55 | 15.70 | 51 | Sarsasapogenin | -9.58 | 0.095 |
| 25 | Deguelin | -7.31 | 4.36 | 52 | Sclareol | -8.43 | 0.662 |
| 26 | Galangin | -6.50 | 17.27 | 53 | Tanshinone | -7.51 | 3.10 |
Table 2: Results of docking depicting binding energy and Ki of selected secondary metabolites (alkaloids, flavonoids and terpenoids) with TK domain of VEGFR-1.
Active site mapping of docked complex of TK domain of VEGFR-1 with selected alkaloids, flavonoids and terpenoids.
The interaction of selected alkaloids, flavonoids and terpenoids with the TK domain of VEGFR-1, at the active site within 5A°, was analysed and compared. Results in terms of interacting residues, hydrogen bond formation, their distance and atoms involved are shown in Tables 3-5 for alkaloids, flavonoids and terpenoids, respectively. It is noteworthy that two interacting residues namely, Cys1039 and Asp1040, which were found to be common to the drugs (Table 1), were also found to be common for all the eighteen docked alkaloids (Table 3). Furthermore, seven alkaloids namely, corydine, eleuthrin, glaucine, lunacridine, lycorine, noscapine and oxostephanine exhibited hydrogen bond interactions of varying numbers, while other eleven alkaloids, including liriodenine, were found to exhibit none. It is noteworthy that liriodenine, the most potent alkaloid, exhibited other interactions such as π-anion, π-alkyl and van der Waals (Figure 1B).
| S.No | Alkaloids | Interacting residues at the active site | Hydrogen donor atom | Hydrogen acceptor atom |
Distance(A°) |
|---|---|---|---|---|---|
| 1 | Anonaine | Ile 1019, His 1020, Cys 1039, Asp 1040 | - | - | - |
| 2 | Aristolactam | Ile 1038, Cys 1039, Asp 1040 | - | - | - |
| 3 | Canthin-6-one N-oxide | Val 891, Val 892, 1018, Ile 1019, His 1020, Ile 1038, Cys 1039, Asp 1040 | - | - | - |
| 4 | Coptisine | Ile 1019, His 1020, Cys 1039, Asp 1040, Phe 1041 | - | - | - |
| 5 | Corydine | 1018, Ile 1019, His 1020, Cys 1039, Asp 1040,Phe 1041, Gly 1042, Leu 1043 | LYS861:NZCorydine:H | Corydine:OASP1040:O | 3.29993, 2.11225 |
| 6 | Crebanine | 1018, Ile 1019, His 1020, Ile 1038, Cys 1039, Asp 1040, | - | - | - |
| 7 | Dehydrocorydaline | Val 891, Val 892, Ile 1019, His 1020, Ile 1038, Cys 1039, Asp 1040 | - | - | - |
| 8 | Dicentrine | Cys 1018, Ile 1019, His 1020, Cys 1039, Asp 1040 | - | - | - |
| 9 | Eleuthrin | Ile 1038, Cys 1039, Asp 1040 | CYS1018:SG | Eleuthrin:O | 3.54168 |
| 10 | Glaucine | Cys 1018, Ile 1019, His 1020, Cys 1039, Asp 1040 | CYS1018:SG | Glaucine:O | 2.71085 |
| 11 | Liriodenine | Ile 1038, Cys 1039, Asp 1040 | - | - | - |
| 12 | Lunacridine | Ile 1038, Cys 1039, Asp 1040 | ASP1040:N Lunacridine:H | Lunacridine:O ASP 1040:OD1 |
2.9648, 1.76345 |
| 13 | Lycorine | Ile 1019, His 1020, Ile 1038, Cys 1039, Asp 1040 | ARG 1021:NH2 Lycorine:H Lycorine:H | Lycorine:O ASP 1040:OD1 ILE 1019:O | 3.19148, 1.89336, 1.79408 |
| 14 | Noscapine | Ile 1019, His 1020, Arg 1021, Ile 1038, Cys 1039, Asp 1040 | ARG 1021:NH2 | Noscapine:O | 3.32611 |
| 15 | Oliveroline | Cys 1039, Asp 1040, Phe 1041, Gly 1042 | - | - | - |
| 16 | Oxostephanine | Cys 1039, Asp 1040 | CYS1018:SG | Oxostephanine:O | 3.33203 |
| 17 | Piperine | Cys 1039, Asp 1040, Phe 1041 | - | - | - |
| 18 | Protopine | Cys 1039, Asp 1040, Phe 1041 | - | - | - |
Table 3: Interacting residues, hydrogen bond formation, their distance and atoms involved in binding of selected alkaloids at the active site of TK domain of VEGFR-1.
In case of flavonoids, the two interacting residues namely, Cys1039 and Asp1040 were found to be common for most of the flavonoids except glabiridin, rotenolone, rotenone, sinensetin and tephrosin (Table 4). Glabridin, the most potent flavonoid, was found to interact with six residues namely, Ala859, Val 860, Lys861, Val907, Ile908, Val909 and exhibited three H-bond interactions with Ala859, Val907and Glu878 (Figure 1C). The remaining flavonoids, except sinensetin, also exhibited H-bond interactions of varying numbers. Glabridin and sinensetin interacted in a similar manner as residues namely, Ala859, Val860 and Lys861 were common amongst them. In case of rotenolone, rotenone and tephrosin, residues namely, Gly1042, Leu1043, Ala1044, Arg1045, Asp1046, Ile1047, Tyr1048 were found to be common amongst them. All the flavonoids were found to interact with hydrogen bonds except sinensetin which exhibited π-bonds and van der Waals interactions.
Similar to alkaloids and flavonoids, terpenoids were also found to be interacting with the TK domain in a similar fashion, as it is revealed by the data in Table 5. All the nine terpenoids exhibited common residues namely, Cys1039 and Asp1040 similar to alkaloids (Table 3), flavonoids (Table 4) and drugs (Table 1) as well. Except the terpenoids, limonene and tanshinone, all the other terpenoids were found to be involved in hydrogen bond interaction. Sarsasapogenin, the most potent flavonoid, interacted with total five residues namely, Thr877, Glu878, Ile1038, Cys1039, Asp1040. However, sarsasapogenin only exhibited two H-bond interaction with residues namely, Ala874 and Ile1038 (Figure 1D). Except limonene and tanshinone, the remaining terpenoids exhibited H-bond interactions of varying numbers.
| S.No | Flavonoids | Interacting residues at the active site | Hydrogen donor atom | Hydrogen acceptor atom | Distance(A°) | |
|---|---|---|---|---|---|---|
| 1 | Acacetin | Val 891, Val 892, Ile 1019, His 1020, Cys1039, Asp 1040, Phe 1041 | Acacetin:H | ASP 1040:O | 2.017 | |
| 2 | Apigenin | Val 891, Val 892, Ile 1019, His 1020, Ile 1038, Cys 1039, Asp 1040, Phe 1041 | Apigenin:HApigenin:HApigenin:HApigenin:H | ILE1019:O ILE1038:O GLU878:OE2 ASP 1040:O |
1.82215 2.11495 2.92904 1.93145 | |
| 3 | Baicalein | Val 891, Val 892, Ile 1038, Cys 1039, Asp 1040 | Baicalein:HBaicalein:H | ILE 1038:O ILE 1038:O | 2.02079 2.26283 | |
| 4 | Cajanol | Ile 1019, His 1020, Cys 1039, Asp 1040 | Cajanol:H | ILE 1019:O | 1.87604 | |
| 5 | Chrysin | Ile 1038, Cys 1039, Asp 1040, Phe 1041 | Chrysin:H Chrysin:H Chrysin:H | ILE 1038:O GLU 878:OE2 ASP 1040:O |
2.15211 2.79743 2.06705 | |
| 6 | Curcumin | Ile 1019, His 1020, Ile 1038, Cys 1039, Asp 1040 | Curcumin:H | ILE 1019:O | 2.00349 | |
| 7 | Deguelin | His 1020, Arg 1021, Asp 1022, Ile 1038, Cys 1039, Asp 1040 | ARG1021:NE | Deguelin:O | 3.1772 | |
| 8 | Galangin | Ile 1038, Cys 1039, Asp 1040,Phe 1041 | CYS1018:SG Galangin:H Galangin:HGalangin:H | Galangin:O ILE1038:O GLU878:OE2 ASP1040:O | 3.17445 2.16006 2.74078 2.07247 | |
| 9 | Genkwanin | Val 891, Val 892, Ile 1019, His 1020, Ile 1038, Cys 1039, Asp 1040 | Genkwanin:H Genkwanin:H |
ILE1019:O ILE 1038:O | 1.87614 2.15797 | |
| 10 | Glabridin | Ala 859, Val 860, Lys 861, Val 907, Ile 908, Val 909 | Glabridin:HGlabridin:HGlabridin:H | ALA859:O VAL907:O GLU878:OE2 | 2.55172 2.25974 1.88071 | |
| 11 | Helichrysetin | Val 891, Val 892, Ile 1019, His 1020, Ile 1038, Cys 1039, Asp 1040, Phe 1041 | Helichrysetin:H Helichrysetin:H Helichrysetin:H | ILE1019:O ILE 1038:O ASP 1040:O | 1.82937 1.89417 2.06257 | |
| 12 | Lethedocin | Val 891, Val 892, Cys 1018, Ile 1019, His 1020, Cys 1039, Asp 1040 | CYS1018:SG Lethedocin:H | Lethedocin:OILE 1019:O | 3.15792 2.0246 | |
| 13 | Licochalcone | Cys 1018, Ile 1019, His 1020, Arg 1021, Cys 1039, Asp 1040, Phe 1041 | Licochalcone:H Licochalcone:H | ASP 1040:O ILE 1019:O | 2.28442 1.77287 | |
| 14 | Morindone | Cys 1018, Ile 1019, His 1020, Cys 1039, Asp 1040, Phe 1041 | CYS1018:SG, CYS 1018:SG Morindone:H Morindone:H Morindone:H | Morindone:OMorindone:OASP 1040:O ILE 1019:O ILE 1019:O | 3.57105 3.11884 2.14614 1.70721 1.87148 | |
| 15 | Mucronulatol | Ile 1019, His 1020, Ile 1038, Cys 1039, Asp 1040 | Mucronulatol:H Mucronulatol:H | ILE 1038:O ILE 1019:O | 2.07534 2.029212 | |
| 16 | Nobiletin | Ala 859, Val 860, Lys 861, Ile 1038, Cys 1039, Asp 1040,Phe 1041 | VAL 892:N | Nobiletin:O | 3.31694 | |
| 17 | Pongavilleanine | Cys 1039, Asp 1040,Phe 1041, Gly 1042, Leu 1043 | LYS861:NZ | Pongavilleanine:O | 2.94262 | |
| 18 | Pseudobaptigenin | Ile 1019, His 1020, Cys 1039, Asp 1040 | Pseudobaptigenin:H | ILE 1019:O | 1.7829 | |
| 19 | Rotenolone | Gly 836, Ala 837, Phe 838, Gly 839, Gly 1042, Leu 1043, Ala 1044, Arg 1045, Asp 1046, Ile 1047, Tyr 1048 | ILE1047:N TYR1048:NRotenolone:H | Rotenolone:O Rotenolone: O LEU 1043:O |
2.99641 3.17141 2.21351 | |
| 20 | Rotenone | Gly 1042, Leu 1043, Ala 1044, Arg 1045, Asp 1046, Ile 1047, Tyr 1048 | ARG1045N ILE 1047:N | Rotenone:O Rotenone:O | 3.28795, 3.03127 | |
| 21 | Sinensetin | Ala 859, Val 860, Lys 861 | - | - | - | |
| 22 | Tangeretin | Cys 1018, Ile 1019, His 1020, Cys 1039, Asp 1040 | CYS1018:SG | Tangeretin:O | 3.18936 | |
| 23 | Tephrosin | Gly 836, Ala 837, Phe838,Gly 1042, Leu 1043, Ala 1044, Arg 1045, Asp 1046, Ile 1047, Tyr 1048 | ARG1045NILE1047:N TYR1048:NTephrosin:H | Tephrosin:OTephrosin:OTephrosin:O LEU 1043:O | 3.28455, 2.90119, 3.15989, 2.15608 | |
| 24 | Velutin | Ile 1019, His 1020, Cys 1039, Asp 1040, Phe 1041 | Velutin:H | ILE 1019:O | 2.21837 | |
| 25 | Wogonin | Ile 1019, His 1020, Ile 1038, Cys 1039, Asp 1040, Phe 1041 | LYS861:NZWogonin:HWogonin:HWogonin:H | Wogonin:O GLU876:OE2 ASP 1040:O ILE 1038:O | 3.25402, 2.74851, 1.96777, 2.15419 | |
| 26 | Zapotin | Cys 1039, Asp 1040 | ASP1040:N | Zapotin:O4 | 3.16682 | |
Table 4: Interacting residues, hydrogen bond formation, their distance and atoms involved in binding of selected flavonoids at the active site of TK domain of VEGFR-1.
Thus, from the data presented in Tables 3-5, it is evident that all the secondary metabolites (alkaloids, flavonoids and terpenoids) are binding at more or less same binding pocket within the active site of the TK domain of VEGFR-1, as revealed by the fact that majority of the interacting residues are common.
| S.No | Terpenoids | Interacting residues at the active site | Hydrogen donor atom | Hydrogen acceptor atom | Distance(A°) |
|---|---|---|---|---|---|
| 1 | Carnosol | Ile 1038, Cys 1039, Asp 1040 | Carnosol:H Carnosol:H | HIS1020:O HIS 1020:O | 2.12813 2.60024 |
| 2 | Cynaropicrin | Ile 1038, Cys 1039, Asp 1040, Phe 1041 | ARG1021:NH2 Cynaropicrin :H Cynaropicrin :H |
Cynaropicrin:O ILE 1019:O ASP 1040:O | 3.03039 1.92452 2.76579 |
| 3 | Limonene | Cys 1039, Asp 1040, Phe 1041 | - | - | - |
| 4 | MansononeE | Cys 1039, Asp 1040 | LYS 861:NZ | MansononeE:O | 3.36718 |
| 5 | Menthol | Val 891, Val 892,Asn 893, Ile 1038, Cys 1039, Asp 1040 | Menthol:H | VAL 892:O | 1.74392 |
| 6 | Salvicine | Ile 1019, His 1020, Ile 1038, Cys 1039, Asp 1040 | CYS1018:SGARG1021:NE ARG1021:NH2 Salvicine:H Salvicine:H | Salvicine:O Salvicine:O Salvicine:O ILE 1019:O HIS 1020:O | 3.15973 2.94908 3.31484 2.16707 2.12479 |
| 7 | Sarsasapogenin | Thr 877, Glu 878, Ile 1038, Cys 1039, Asp 1040 | Sarsasapogenin:H Sarsasapogenin:C | ILE1038:O ALA 874:O | 1.8845 3.77462 |
| 8 | Sclareol | Ile 1019, His 1020, Arg 1021, Ile 1038, Cys 1039, Asp 1040 | ARG1021:NESclareol:HSclareol:H | Sclareol:O ILE 1019:O ASP1040:OD1 | 3.09214, 1.90063, 2.09362 |
| 9 | Tanshinone | Ile 1038, Cys 1039, Asp 1040 | - | - | - |
Table 5: Interacting residues, hydrogen bond formation, their distance and atoms involved in binding of selected terpenoids at the active site of TK domain of VEGFR-1.
MD simulation analyses
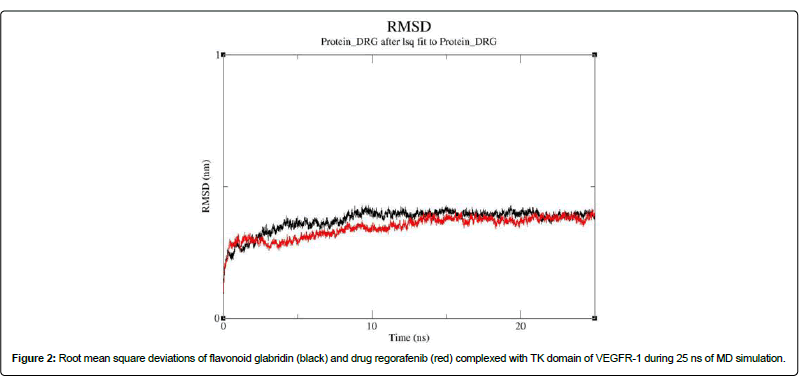
The stability of the best docked complex of flavonoid glabridin with that of TK-VEGFR-1 was further validated by MDS analyses. For comparison, the best docked drug regorafenib complexed with TK domain of VEGFR-1 was also analysed by MDS analyses under similar conditions. The results of MDS analyses for 25 ns are presented in (Figure 2). It is noteworthy that the average root mean square deviation (RMSD) values of the flavonoid glabridin complexed with TK domain of VEGFR-1 was found to be 0.34 nm and for that of drug regorafenib was found to be 0.30 nm. Thus, the data revealed that the complex of flavonoid glabridin was stabilized around 10 ns of simulation while that of drug regorafenib was stabilized around 20 ns of simulation.
Binding free energy analyses
The stability of the docked complexes of flavonoid glabridin and drug regorafenib with TK domain of VEGFR-1, is further analysed by calculating free binding energies using MM/PBSA (molecular mechanics Poisson-Boltzmann surface area) method. Results of the analyses of the above mentioned complexes, for the 20-25 ns of MD simulation are presented in Table 6. From the Table 6, it can be concluded, that flavonoid glabridin and drug regorafenib, complexed with TK domain of VEGFR-1, exhibited binding energy (ΔGbind) of -203.932 kJ/Mol and -177.632 kJ/Mol, respectively. Thus, based on these observations, it can be concluded that MM/PBSA binding free-energy analyses corroborated well with the results of molecular docking and MD simulation analyses, and revealed significantly lower binding energy for drug regorafenib as compared to that of flavonoid glabridin. Thus, based on analyses conducted in the present study, it may be concluded that the flavonoid, glabridin, qualifies for its further testing, using in vivo/in vitro studies, to develop as a potential anti-angiogenic drug.
| Energy components | Drug (Regorafenib) | Flavonoid (Glabridin) |
|---|---|---|
| ΔEvdwa | -274.926 | -228.753 |
| ΔEeleb | -89.949 | -5.839 |
| ΔGpolc | 208.997 | 47.629 |
| SASA energyd | -21.753 | -16.968 |
| ΔGbinde | -177.632 | -203.932 |
| avan der Waals interaction energies. bElectrostatic interaction energies. cPolar solvation free energy. dSolvent-accesible surface area eBinding energies |
||
Table 6: Binding energies (kJ/mol) for drug regorafenib and flavonoid glabridin complexed with TK-VEGFR-1, during 20-25 ns (equilibrium phase) of MD simulation trajectory.
Discussion
VEGFR signalling is tightly regulated at different levels such as receptor expression, the availability and affinities for binding of its different ligands, the presence of VEGF-binding co-receptors, non-VEGF-binding auxiliary proteins and inactivating tyrosine phosphatases. Targeting TK domain of VEGFR-1 has been considered as a promising approach in cancer therapeutics. VEGFR-1 small kinase inhibitors (drugs) are more effective along with the combination therapies. Furthermore, in preclinical studies, various FDA approved drugs directed against VEGFR-1, have been reported to block antitumor activity. Small-molecule inhibitors are largely hydrophobic and can easily enter the cell where they can interact with the intracellular domain of receptors and thereby, block the activation of various downstream signalling pathways intracellularly. The small-molecule tyrosine kinase inhibitors namely, axitinib, lenvatinib, pazopanib, regorafenib, sorafenib, sunitinib, vandetanib, are all noncovalent ATP competitive inhibitors, inactivate all VEGFRs and are considered as multi-targeted agents since they inhibit several tyrosine kinase growth factor receptors (e.g., c-KIT, platelet derived growth factor and FGF receptors). In literature, regorafenib, a multi-kinase inhibitor, has been reported to efficiently inhibit tumor growth and angiogenesis in both preclinical and clinical phase I to III trials [44,45]. Regorafenib, potently inhibits other angiogenic and stromal factors such as, TIE2 and PDGFR-b that contribute in tumor neovascularization, vessel stabilization and lymphatic vessel formation and play an important role in the tumor microenvironment, which ultimately leads to tumor development and metastasis formation [46,47]. Furthermore, sunitinib, a type- 1 tyrosine kinase inhibitor, targeting VEGFR-1 and 3, has been reported to block PDGFR-α, KIT, fms-related tyrosine kinase 3 (FLT- 3), colony stimulating factor-1 receptor (CSF-1R) [48].
There is a worldwide demand for safer alternative therapeutic molecules coming from natural sources in view of the current synthetic drugs targeting TK domain of VEGFR-1 exhibiting severe side effects during their long term use [20]. In this context, the present work deals with identification of potent inhibitors of TK domain of VEGFR-1 coming from plant derived secondary metabolites. Thus, in the present paper, anti-angiogenic potential of ADMET screened 18 alkaloids, 26 flavonoids and 9 terpenoids, targeted against TK domain of VEGFR-1, have been investigated through molecular docking approach. Results of the analyses revealed that the alkaloid liriodenine, flavonoid glabridin and terpenoid sarsasapogenin were found to be the best among each class of secondary metabolites analysed. However, across all the categories of the analysed secondary metabolites, flavonoid glabridin was found to be the most potent inhibitor. Liriodenine, the most potent alkaloid has been reported in literature, to induce DNA damage, suppress the expression of cyclin D1 and cyclin-dependent kinase and decreased phosphorylation of retinoblastoma protein in tumor cells leading to G1/S phase arrest [49-52].
The flavonoid glabridin has been reported to exhibit growth inhibition of a number of cancers in humans [53]. In addition, it has also been reported to exhibit multiple biological properties, such as antibacterial, neuroprotective, antiatherosclerotic, antiosteoporotic and immunomodulatory [54,55]. Because of its other beneficiary properties, glabridin also finds its applications in cosmetics and food industries. The glabridin is found in the roots of Glycyrrhiza glabra, commonly known as licorice and is readily available worldwide.
The most potent terpenoid, sarsasapogenin has been reported to exhibit biological effects such as antimicrobial, anti-inflammatory, anti-proliferative and anti-apoptotic in various cancer cell lines. In literature, sarsasapogenin has been reported to be a potent inhibitor of the proliferation of human 1547 osteosarcoma cells and to arrest cell cycle in G2/M phase [56]. Sarsasapogenin has been reported to be present in many dietary products such as asparagus, herbs and spices and fenugreek (Indian spice).
In order to compare anti-angiogenic potential of these secondary metabolites, results of docking has been compared with those of well-known anti-angiogenic drugs directed against VEGFR-1. Thus, among the ten FDA approved drugs, regorafenib was found to be the best. It is also noteworthy, that the alkaloids, flavonoids and terpenoids in comparison to drugs, have interacted more or less at the same binding pocket of the TK domain of VEGFR-1 as evident from the results. Furthermore, the interacting residues at the active site namely Cys1039 and Asp1040 are common across the categories of secondary metabolites as well as drugs. In addition, the binding affinity of flavonoids and terpenoids with the TK domain of VEGFR-1, in comparison to alkaloids, was better as revealed by the docking scores. Also it is noteworthy, that the drug lenalidomide and flavonoid licochalcone exhibited same binding energy. In addition, some flavonoids like tephrosin, sinensetin, nobiletin, genkwanin and apigenin exhibited better binding energy in comparison to drugs such as axitinib, lenalidomide, motesanib, pegaptanib and vatalanib. Similarly, terpenoids, such as sclareol, cynaropicrin and carnosol exhibited comparable binding energy to drugs as stated above.
However, with the long term use of the aforesaid drugs, certain toxicities have been observed like hypertension, bleeding, fatigue, diarrhoea, nausea and/or vomiting, hand foot syndrome, and myelosuppression [57,58]. Therefore, there is a need to look for safer alternatives in cancer therapy. It can be concluded that the secondary metabolite stated above can disrupt the downstream signalling of VEGFR-1, hence blocking angiogenesis. Also, the plant derived, secondary metabolites can provide safer alternative as compared to drugs without causing any side effects to the individuals.
Conclusion
Angiogenesis, one of the hallmarks of cancer, eventually leads to the formation of solid tumors. Targeting tumor angiogenesis has been one of the common approaches in cancer therapeutics. In the recent years, TK domain of VEGFR-1 has been exploited by researchers worldwide for its anti angiogenic potential. Therefore, any therapeutic approach to identify novel inhibitor against the TK domain of VEGFR-1 will be a key lead in the development of anti angiogenic drugs. Furthermore, the long term use of the synthetic drugs has been reported to develop toxicities and ill effects in individuals. Therefore, in cancer therapeutic interventions, there is a need to develop safer drugs, coming from natural sources as they will have little or no side effects. In the present paper, we have evaluated the inhibitory potential of ADMET screened secondary metabolites, belonging to alkaloids (18), flavonoids (26) and terpenoids (9), against the TK domain of VEGFR-1, by molecular docking approach and compared the results with those of FDA approved drugs. Results of docking, revealed that out of the ten drugs analysed, the most potent was found to be regorafenib. Among all the alkaloids, flavonoids and terpenoids analysed, liriodenine, glabridin, sarsasapogenin, respectively, were found to be best category wise. However, among all the secondary metabolites taken together, flavonoid glabridin was found to be most potent inhibitor of the TK domain of VEGFR-1. Thus, the present study makes a foundation for further investigations based on the wet lab experiments for therapeutic applications of screened secondary metabolites as anticancer drugs in general and glabridin and sarsasapogenin, in particular, as potent anti angiogenic molecules.
Conflict of Interest
The author declares that no conflict of interest exists.
Acknowledgment
Financial supports from Department of Higher Education, Government of U.P towards the establishment of Institute for Development of Advanced Computing and under Centre of Excellence Grant are gratefully acknowledged. Also financial supports from Department of Biotechnology (DBT), New Delhi under Bioinformatics Infrastructure Facility and Department of Science and Technology, New Delhi under Promotion of University Research and Scientific Excellence are gratefully acknowledged.
References
- Ho VC, Fong GH (2015) Vasculogenesis and angiogenesis in VEGF receptor-1 deficient mice. Mol Biol 1332: 161-176.
- Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L (2011) Signal transduction by vascular endothelial growth factor receptors. Biochem J 437: 169-183.
- Cartland SP, Genner SW, Zahoor A, Kavurma MM(2016) Comparative evaluation of trail, FGF-2 and VEGF-a-induced angiogenesis in vitro and in vivo. Int J Mol Sci17: 12.
- De-Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, et al (1992) The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor.Science255: 989-991.
- Ferrara N, Davis-Smith T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18: 4-25.
- Shibuya M, Yamaguchi S, Yamane A, Ikeda T, Tojo A, et al (1990) Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 5: 519-524.
- Keyt BA, Nguyen HV, Berleau LT, Duarte CM, Park J, et al (1996) Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors, generation of receptor-selective VEGF variants by site-directed mutagenesis. J BiolChem 271: 5638-5646.
- Tanaka K, Yamaguchi S, Sawano A Shibuya M (1997) Characterization of the extracellular domain in the Vascular Endothelial Growth Factor Receptor-1 (Flt-1 tyrosine kinase). Jpn J Cancer Res 88: 867-876
- Schlessinger J, Lemmon MA (2000) Cell signaling by receptor tyrosine kinases. Cell 103: 211-225.
- Tsai CJ, Nussinov R (2013) The molecular basis of targeting protein kinases in cancer therapeutics.Semin Cancer Biol 23: 235-242.
- Weddell JC, Imoukhuede PI (2014) Quantitative characterization of cellular membrane-receptor heterogeneity through statistical and computational modeling. Plos One 9 e97271.
- Duda DG, Willett CG, Ancukiewicz M, di Tomaso E, Shah M, et al. (2010) Plasma soluble VEGFR-1 is a potential dual biomarker of response and toxicity for bevacizumab with chemoradiation in locally advanced rectal cancer. Oncologist 15: 577-583.
- Saha S, Islam MK, Shilpi JA, Hasan S (2013) Inhibition of VEGF: A novel mechanism to control angiogenesis byWithania somnifera’skey metabolite withaferin A. In Silico Pharmacol 1: 11-19.
- Kadioglu O, Seo E, Efferth T (2013) Targeting angiogenesis by phytochemicals. Med Aromat Plants 2: 1‑8.
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engh J Med 350: 2335-2342.
- Bupathi MK, Ahn DH, Bekaii-Saab T (2016) Spotlight on bevacizumab in metastatic colorectal cancer: patient selection and perspectives. Gastrointest Cancer 6: 21-30
- Whyte S, Pandor A, Stevenson M, Rees A (2010) Bevacizumab in combination with fluoropyrimidine-based chemotherapy for the first-line treatment of metastatic colorectal cancer. Health Technol Assess 14: 47-53
- Woo HY, Heo J (2012) Sorafenib in liver cancer. Exp Opin Pharmacother 13: 1059-1067
- Roskoski R Jr (2007) Sunitinib: A VEGF and PDGF receptor protein kinase and angiogenesis inhibitor. Biochem Biophys Res Commun 356: 323-328
- Wilmes LJ, Pallavicini MG, Fleming LM, Gibbs J, Wang D, et al. (2007) AG-013736, a novel inhibitor of VEGF receptor tyrosine kinases, inhibits breast cancer growth and decreases vascular permeability as detected by dynamic contrast-enhanced magnetic resonance imaging. Magn Reson Imaging 25: 319-327
- Bukowski RM, Yasothan U, Kirkpatrick P (2010) Pazopanib. Nat Rev Drug Disc 9: 17-18
- Verheul HM, Pinedo HM (2007) Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition.Nat Rev Cancer7: 475-485
- Suffness M. (1995)Taxol: Science and Applications.
- Leon P, Garbay-Jaureguiberry C, Barsi MC, Le Pecq JB, Roques BP (1987) Modulation of the antitumor activity by methyl substitutions in the series of 7H-pyridocarbazole monomers and dimers. J Med Chem 30: 2074-2080
- Russell EG, O'Sullivan EC, Miller CM, Stanicka J, McCarthy FO, et al. (2014) Ellipticine derivative induces potent cytostatic effect in acute myeloid leukaemia cells. Invest New Drugs 32: 1113-1122
- Aggarwal BB, Kumar A, Bharti AC (2003)Anticancer potential of curcumin: Preclinical and clinical studies.Anticancer Res23: 363-398
- Chatterjee SJ, Pandey S (2011)Chemo-resistant melanoma sensitized by tamoxifen to low dose curcumin treatment through induction of apoptosis and autophagy.Cancer Biol Ther11: 216-228
- Arbiser JL,Klauber N,Rohan R,van Leeuwen R,Huang MT,et al. (1998) Curcumin is an in vivo inhibitor of angiogenesis. Mol Med4: 376-383.
- Schindler R, Mentlein R (2006) Flavonoids and vitamin E reduce the release of the angiogenic peptide vascular endothelial growth factor from human tumor cells. J Nutr 136: 1477‑1482.
- Bagli E, Stefaniotou M, Morbidelli L, Ziche M, Psillas K, et al. (2004) Luteolin inhibits vascular endothelial growth factor‑induced angiogenesis; inhibition of endothelial cell survival and proliferation by targeting phosphatidylinositol 3’‑kinase activity. Cancer Res 64: 7936‑7446.
- Fang J, Xia C, Cao Z, Zheng JZ, Reed E, et al. (2005) Apigenin inhibits VEGF andHIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J 19: 342-353
- Yu X, Lin H, Wang Y, Lv W, Zhang S, et al. (2018) D-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. Onco Targets Ther 11: 1833-1847.
- Bardon S, Foussard V, Fournel S, LoubatA (2002) Monoterpenes inhibit proliferation of human colon cancer cells by modulating cell cycle-related protein expression. Cancer Lett 181: 187-194.
- Chowdhury AR, Mandal S, Mittra B, Sharma S, Mukhopadhyay S, et al. (2002) Betulinic acid, a potent inhibitor of eucaryotic topoisomerase I; identification of the inhibitory step, the major functional group responsible and development of more potent derivatives. Med Sci Monit 8: 254-265
- Lang JY, Chen H, Zhou J, Zhang YX, Zhang XW, et al. (2005) Antimetastatic effect of salvicine on human breast cancer MDA-MB-435 orthotopic xenograft is closely related to Rho-dependent pathway. Clin Cancer Res 11: 3455-3464.
- Mohan R,Hammers HJ, Bargagna-Mohan P, Zhan XH, Herbstritt CJ, et al. (2004) Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis7: 115-122.
- Ponnan P, Gupta S, Chopra M, Tandon R, Baghel AS, et al. (2013) 2D-QSAR, docking studies, and In Silico ADMET prediction of polyphenolic acetates as substrates for protein acetyltransferase function of glutamine synthetase of Mycobacterium tuberculosis. ISRN Struct Biol 1-12.
- HopCE, Cole MJ, Davidson, Duignan DB, Federico J, et al. (2008) High throughput ADME screening: Practical considerations, impact on the portfolio and enabler of in silico ADME models. Curr Drug Metab 9: 847-853.
- Oprea TI (2002) Virtual screening in lead discovery: A Viewpoint. Molecules 7: 51-62
- O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, et al. (2011) Open Babel: An open chemical tool box. J Cheminform 3: 33.
- Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, et al. (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19: 1639-1662.
- Schuttelkopf AW, van Aalten DM (2004) PRODRG: A tool for high throughput crystallography of protein-ligand complexes. ActaCrystallogr D Biol Crystallogr 60: 1355-1363
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, et al. (2004) UCSF Chimera-a visualization system for exploratory research and analysis. J Comput Chem 25: 1605-1612.
- Mross K, Frost A, Steinbild S, Hedbom S, Buchert M, et al. (2012) A € phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res 18: 2658-2567.
- Strumberg D, Scheulen ME, Schultheis B, Richly H, Frost A, et al. (2012) Regorafenib (BAY 73-4506) in advanced colorectal cancer: A phase I study. Br J Cancer 106: 1722-1727.
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, et al. (2000) Vascular specific growth factors and blood vessel formation. Nature 407: 242-248.
- Augustin HG, Koh GY, Thurston G, Alitalo K (2009) Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 10: 165-77.
- Faivre S, Demetri G, Sargent W, Raymond E (2007) Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov 6: 734-745.
- Hsieh TJ, Liu TZ, Chern CL, Tsao DA, Lu FJ, et al. (2005) Liriodenine inhibits the proliferation of human hepatoma cell lines by blocking cell cycle progression and nitric oxide-mediated activation of p53 expression. Food ChemToxicol 43: 1117-1126.
- Chang HC, Chang FR, Wu YC, Lai YH (2004) Anti-cancer effect of liriodenine on human lung cancer cells. Kaohsiung J Med Sci 20: 365-371
- Chen ZF, Liu YC, Peng Y, Hong X, Wang HH, et al. (2012) Synthesis, characterization, and in vitro antitumor properties of gold (III) compounds with the traditional Chinese medicine (TCM) active ingredient liriodenine. J Biol Inorg Chem 17: 247-261.
- Li YL, Qin QP, Liu YC, Chen ZF, Liang H (2014) A platinum(II) complex of liriodenine from traditional Chinese medicine (TCM): Cell cycle arrest, cell apoptosis induction and telomerase inhibition activity via G-quadruplex DNA stabilization. J Inorg Biochem 137: 12-21.
- Yu XQ, Xue CC, Zhou ZW, Li CG, Du YM, et al. (2008) In vitro and in vivo neuroprotective effect and mechanisms of glabridin, a major active isoflavan from Glycyrrhiza glabra (licorice). Life Sci 82: 68-78.
- Aoki F, Nakagawa K, Kitano M, Hideyuki I, Kenjirou N, et al. (2007) Clinical safety of licorice flavonoid oil (LFO) and pharmacokinetics of glabridin in healthy humans. J Am Coll Nutr 26: 209-218.
- Tamir S, Eizenberg M, Somjen D, Stern N, Shelach R, et al. (2000) Estrogenic and antiproliferative properties of glabridin from licorice in human breast cancer cells. Cancer Res 60: 5704-5709
- Trouillas P, Corbiere C, Liagre B, Duroux JL, Beneytout JL (2005) Structure function relationship for saponin effects on cell cycle arrest and apoptosis in the human 1547 osteosarcoma cells: A molecular modelling approach of natural molecules structurally close to diosgenin. Bioorg Med Chem 13: 1141-1149
- Eskens FA, Verweij J (2006) The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur J Cancer 42: 3127-3139.
- Bhojani N, Jeldres C, Patard JJ, Perrotte P, Suardi N, et al. (2008) Toxicities associated with the administration of sorafenib, sunitinib, and temsirolimus and their management in patients with metastatic renal cell carcinoma. Eur Urol 53: 917-930.