Research Article, Jbhrd Vol: 5 Issue: 2
Association of Epstein-Barr Virus (EBV) Infection with Histomorphological and Immunohistochemical Features of Hodgkin Lymphoma
MVA Sireesha1*, Rajesh Kumar KS2, Kiran PK3, Vinu Sarathy3, Suhail Sayeed Mufti4, Diganta Hazarika4 and RadheshyamNaik51 Department of Pathology, HealthCare Global (HCG) Hospital, Bengaluru, India
2 Department of Translational Medicine & Therapeutics, HealthCare Global (HCG) Hospital, Bengaluru, India
3 Department of Medical Oncology, HealthCare Global (HCG) Hospital, Bengaluru, India
4 Department of Translational Medicine & Therapeutics, HealthCare Global (HCG) Hospital, Bengaluru, India
5 Department of Translational Medicine & Therapeutics; Head, Department of Medical Oncology, Hematology and BMT, Bengaluru, India
*Corresponding Author : MVA Sireesha
DNB Resident, Department of Pathology,
HealthCare Global (HCG) Hospital, Bengaluru, India
Email: mvasirriisha019@gmail.com
Received date: March 30, 2020; Accepted date: April 16, 2020; Published date: April 25, 2020
Citation: Sireesha MVA, Rajesh Kumar KS, Kiran PK, Sarathy V, Mufti SS, et al. (2020) Association of Epstein-Barr Virus (EBV) Infection with Histomorphological and Immunohistochemical Features of Hodgkin Lymphoma. J Blood Res Hematol Dis 5:2. doi: 10.37532/jbhrd.2020.5(2).118
Abstract
Background: Lymphomas are a heterogenous group of lymphoproliferative disorders arising from B-cells, T-cells or Natural Killer (NK) cells, which are classified into Hodgkin and Non-Hodgkin Lymphoma. Hodgkin Lymphoma is categorised into Classical Hodgkin Lymphoma (CHL) and Non Classical types according to clinical, histomorphological, phenotypic and genotypic features. In India, the incidence of lymphoma has increased in the last decade and the increased incidence is found in patients of fifth and sixth decade. The etiology of HL remains unclear, usually arises denovo or seen to arise from Epstein-Barr viral infection. Analyzing expression of abundant EBV small nuclear RNA transcripts (EBER) in RS cells by RNA In Situ Hybridisation (RISH) and immunehistochemical expression of latent membrane protein (LMP1)together are more conclusive for identifying EBV-related Hodgkin Lymphomas than either assay alone. Here we studied etiopathogenic role of EBV in HL.
Methods: This was a hospital based ethics approved study conducted at the Department of Pathology, Health Care Global Specialty Hospital, Bangalore from June 2017 to May 2019.
Results: We found among 57 cases of Hodgkin Lymphoma, 33 were EBER positive, 37 were LMP 1 positive, overall, 41 cases (71.9%) were EBV positive. There was statistically significant association between gender and LMP1 (p=0.037). There was a statistically significant association of EBER with morphological subtypes (p=0.034) and Ann Arbor Staging (p=0.013). EBER expression was more frequently found in mixed cellularity subtype and in advanced stages of Hodgkin Lymphoma.
Conclusion: EBER expression had significant association (p=0.034) with HL subtypes and EBV also had significant association (p=0.041). But, withLMP1 expression alone, there was no significant association (p=0.191) with the subtypes, suggesting analysing expression of both EBER and LMP1 for studying association of EBV in HL.
Keywords: Lymphomas, Genotypic
Introduction
Lymphomas are a heterogenous group of lymphoproliferative disorders arising from B-cells, T-cells or Natural Killer (NK) cells, which are classified into Hodgkin and Non-Hodgkin Lymphoma. In India, 80 to 85% cases are B-cell lymphomas, 15 to 20% cases are T-cell lymphomas and NK cell lymphomas are rare [1]. In India, the incidence of lymphoma has increased in the last decade and the increased incidence is found in patients of fifth and sixth decade.Every year, HL contributes to 1% of all de novo neoplasms that occurs worldwide [2].
The of HL remains unclear, nevertheless careful looking into the unique clinical and epidemiological features, an infectious cause has long been suspected. Usually arises denovo or seen to arise from Epstein-Barr viral infection. Identification of raised antibody titres to EBV antigens in HL patients when compared to other lymphoma patients and previous history of Infectious Mononucleosis in HL patients suggested the first evidence that EBV might have been involved in the pathogenesis of HL [3]. HL is categorised into CHL and Non Classical types according to clinical, histomorphological, phenotypic and genotypic features. Classical Hodgkin Lymphoma (CHL) is the most common type and is sub classified into Mixed cellularity (MC), Nodular Sclerosis (NS), Lymphocyte depleted (LD), Lymphocyte rich variants (LR). MC subtype of CHL is the most commonly associated with Epstein Barr virus. Females are more commonly affected than males.
Epstein Barr Virus is a DNA virus, formally called Human gammaherpesvirus 4, is one of the nine known in the . It is subclassified in to lymphocryptovirus and hence is a most important etiological factor for various lymphoid malignancies such as Hodgkin Lymphoma, Burkitt Lymphoma as well as Infectious mononucleosis, HLH (HaemophagocyticLymphohistiocytosis) syndrome, non- lymphoid malignancies such as nasopharyngeal carcinoma and gastric cancers. Hence our study includes etiopathogenic role of EBV in HL. The oncogenic potential of EBV is mainly attributed to its capability to exist in the latent state within B-lymphocytes [4].These infected B-cells in the germinal center develop into resting memory B-cells along a period of time, allowing the virus to remain in dormant state in B-cells. Occasionally, these re-activate to infect new B-cells [5]. Normally EBV is present in latent form and lytic replication occurs only after reactivation from the latency. During latency, viral DNA resides as an episome inside the nucleus of the host cell and host cell DNA polymerase copies it. During latency, only few of the EBV genes are expressed like EBV nuclear antigen 1 (EBNA1), EBV small nuclear RNA transcripts (EBER) and latent membrane protein (LMP) such as LMP1, LMP2A and LMP 2B and these are usually seen in association with Hodgkin Lymphoma and T-cell NHL. EBV-encoded noncoding RNAs (EBER 1 and EBER 2) are most abundant and transcribed as small noncoding non-polyadenylated RNAs by host RNA Polymerase III [6,7]. Identification of the abundant EBER in HRS cells by RNA In Situ Hybridisation (RISH) provides a sensitive method for detecting latent infection in situ. LMP1 is the only EBV protein with proven oncogenic properties and is strongly expressed by HRS cells. Both the EBER and LMP1 assays together are more conclusive than either assay alone for identifying EBV-related Hodgkin Lymphomas [8]. Immunophenotypically, expression of Epstein Barr Virus (EBV) markers such as Epstein-Barr virus (EBV)-encoded small RNAs (EBER by RISH) and Latent membrane protein 1 (LMP1 by IHC) are associated with advanced stages (Stage III and IV), whereas absence of expression of EBER and LMP 1 are associated with early stages of HL (Stage I and II) [9]. Here, we studied etiopathogenic role of EBV by analysing expression of EBER and LMP-1 markers in Hodgkin Lymphoma.
Materials and Methods
This was a hospital based study approved by Institutional Ethics Committee, which was carried out at the Department of Pathology, Health Care Global Specialty Hospital, Bangalore from June 2017 to May 2019. The study group comprised of 57 Hodgkin Lymphoma patients presenting to HCG Hospital, Bangalore. Patients included in this study were biopsy-positive Hodgkin Lymphoma cases who were untreated and newly diagnosed. A detailed history including age, gender, clinical presentation, location of tumor, details of clinically assessed lymph nodes, clinical staging, presence of extra nodal sites were noted from patient medical records. Excision biopsies, trucut biopsies and formalin fixed paraffin embedded blocks of all patients diagnosed as HL are processed and multiple sections of 3-4 micron thickness were taken from paraffin embedded tissue blocks. Then thesections are stained with Hematoxylin & Eosin for studying morphology and subtyping and further taken for immunohistochemical analysis of EBER, LMP1 & CD15. Additional immunohistochemical analysis was also done for CD20, CD3, CD15, CD30, PAX5, MUM1, OCT2 marker (data not shown).
Immunohistochemistry (IHC)
IHC was performed on 3-5 micron sections of formalin fixed and paraffin embedded (FFPE) tissues using a one step (rabbit monoclonal antibody) and two step (mouse monoclonal antibody) polymer-HRP detection system. The primary monoconal antibodies used for IHC are viz., CD20(L26, mouse monoclonal antibody), CD3 (BerH2 + Con6D/B5, mouse monoclonal antibody), CD30 (BerH2 + Con6D/B5, mouse monoclonal antibody), CD15 (MMA, mouse monoclonal antibody), OCT2 (Oct-207, mouse monoclonal antibody), PAX5 (BC/24, mouse monoclonal antibody), MUM1 (BC5, rabbit monoclonal antibody) and LMP1 (EBV01, 02, and 03, mouse monoclonal antibody). Reactive lymph node tissues were used as positive control for the expression of CD20, CD3, OCT2, PAX5 and MUM1 markers; tissue sections from known case of Hodgkin Lymphoma were used positive control for CD30, CD15 and LMP1 markers.
Tissues sectioned, mounted on charge slides and dewaxed. Further slides were treated with an antigen retrieval solution [EDTA buffer (1Mm, pH 8)] if required, blocked with a proteinaceous blocking solution (3% BSA) and then incubated with the primary antibody. The bound primary antibody was detected by the addition of secondary antibody conjugated with horseradish peroxidise polymer and DAB substrate. When adequate colour developed, the slides were washed in water to stop the reaction, counterstained with Harris Haematoxylin, and covered with a mounting medium (DPX). Semi-automated method, Intellipathautostainer was used to stain slides.
In RNA in situ hybridization RISH (procedure, tissues sectioned, mounted on charge slides and dewaxed. Further slides were treated with an antigen retrieval solution [EDTA buffer (1Mm, pH 8)] followed by Hydrogen Peroxidase to block endogenous peroxidase enzymes (primary block) and Sniper to reduce the background staining (secondary block). Subsequently, it was treated with RISH zyme for enzyme digestion. EBER probe (digoxigenin labelled probe from BIOCARE) was added and incubated by placing a coverslip. Secondary and tertiary probes were added followed by a DAB substrate. After adequate colour development, slides were washed in water to stop the reaction, counterstained with Harris Haematoxylin, and covered with a mounting medium (DPX). Semi-automated method, Intellipathautostainer was used to stain slides.
Immunohistochemical interpretation
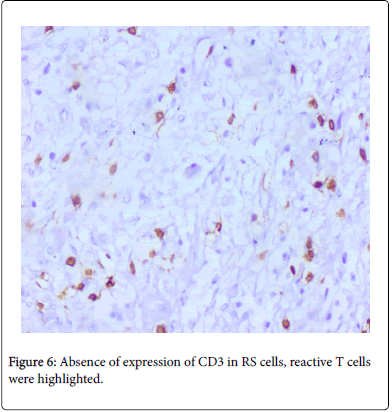
EBER & LMP-1 expressions were considered to be considered as positive when >10% of cells shows the nuclear and membrane expression respectively as described previously. 4TheIHCresultsforCD30, CD20 and CD 15 were designated as positive or negative according to the criteria designated by Dinand et al. and Tzankov et al. respectively with a cut off level of ≥10% [10,11]. MUM1 shows predominantly nuclear staining with occasional weak to moderate cytoplasmic staining [12]. CD3 do not highlight any RS cells and it highlights only background reactive T-lymphocytes. OCT2 expression was considered positive when RS cells express nuclear staining [13]. PAX5 expression was considered low as it shows a weak nuclear expression in RS cells compared to strong nuclear expression of background reactive lymphocytes [14].
Statistical methods
In descriptive statistics, frequency and percentage is used for categorical data analysis. In Inferential statistics, Chi square test were used to assess the association between categorical variables and a Pvalue of <0.05 was considered as statistically significant. SPSS VERSION 23 for windows was used for data analysis.
Results
Patient Clinical Information
A total of 57 patients were included in our study and all of the patients’ data in this study were analyzed for various demographical, clinicopathlogical and histological characteristics as mentioned in the Table 1.
| CHARACTERISTICS | NUMBER (N=57) | PERCENTAGE (%) |
|---|---|---|
| AGE | ||
| <20 years | 9 | 15.8 |
| 20-29 years | 17 | 29.8 |
| 30-39 years | 6 | 10.5 |
| 40-49 years | 6 | 10.5 |
| 50-59 years | 8 | 14 |
| 60-69 years | 6 | 10.5 |
| ≥ 70 years | 5 | 8.8 |
| GENDER | ||
| Male | 39 | 68.4 |
| Female | 18 | 31.6 |
| HL SUB-TYPES | ||
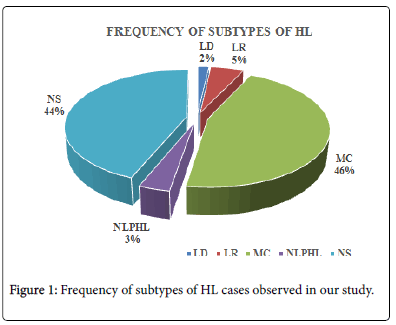
| Lymphocyte Depleted (LD) | 1 | 1.8 |
| Lymphocyte Rich (LR) | 3 | 5.3 |
| Mixed Cellularity (MC) | 26 | 45.6 |
| NLPHL | 2 | 3.5 |
| Nodular Sclerosis (NS) | 25 | 43.9 |
| DISTRIBUTION OF HL CASES (based on the ANN ARBOR STAGING) | ||
| Stage I | 4 | 7 |
| Stage II | 14 | 24.6 |
| Stage III | 23 | 40.4 |
| Stage IV | 16 | 28.1 |
| CD30 AND CD15 POSITIVITY | ||
| CD30 positive | 57 | 100 |
| CD15 positive | 55 | 96.5 |
Table 1: Distribution of Age, Gender, HL subtypes, Ann-Arbor staging and CD30 & CD15 positivity in our study population.
Our study included patients from 5year old patient to 86 year old patient and we could see that more number of subjects was in the age group of 20-29 (29.8%) and even 14.0% of them were 50-59 years age group. The mean age of the subjects was 39.36 with the standard deviation of 20.66 and the median was 36. Total number of males was 39(68.4%) and the females were 18(31.6%). Hodgkin Lymphoma was more seen in males compared to females. In our study we found out of 57 cases, 55 were CD15 positive (96.5%) and 2 were negative (3.5%). All 57 cases were positive to CD30.
Among the subtypes, nearly 45.6% cases were MC subtype and 25 subjects (44%) were NS. Minimum number of subjects was seen in LD subtype (1.8%) and NLPHL (3.5%) (Figure 1).
Histology and Immunohistochemical staining pattern
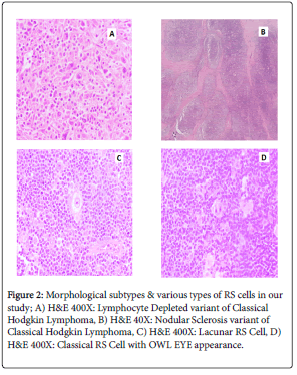
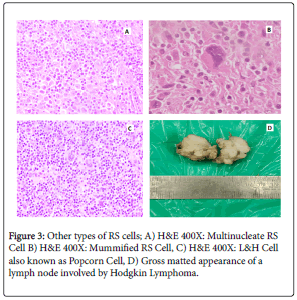
Histomorphological subtypes & various types of RS cells is represented in Figures 2 & 3.
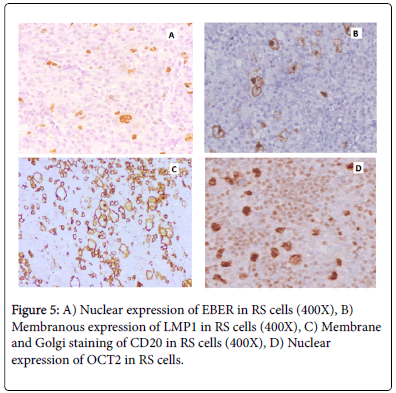
Figure 2: Morphological subtypes & various types of RS cells in our study; A) H&E 400X: Lymphocyte Depleted variant of Classical Hodgkin Lymphoma, B) H&E 40X: Nodular Sclerosis variant of Classical Hodgkin Lymphoma, C) H&E 400X: Lacunar RS Cell, D) H&E 400X: Classical RS Cell with OWL EYE appearance.
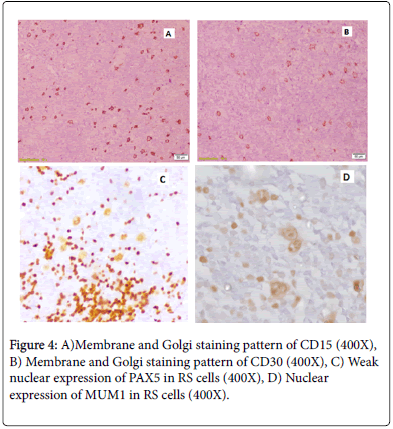
Immunohistochemical staining pattern of CD15, CD30, PAX5 & MUM1 is represented in Figure 4.
Staining pattern of LMP1, EBER, CD20 & OCT2 is shown in Figure 5 and absence of CD3 expression in RS cells (Figure 6).
Frequency of Ann-Arbor Staging distribution in HL cases
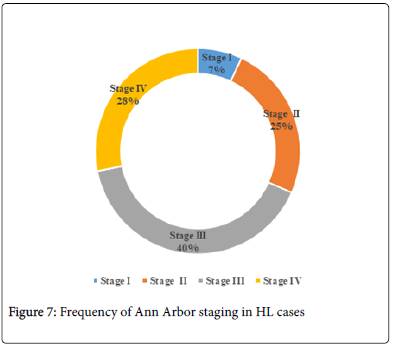
In our study cases, frequency of Stage III HL was highest (40.4%) followed with Stage IV (28.1%). And the least was seen in Stage 1 as 7% (Figure 7).
Association of age with EBER, LMP-1 and EBV positivity
Age wise classification with regard to LMP1, EBV and EBER is given in Table 2. In EBER, positivity was seen maximum in >40 years. Nearly 71.4% were in the age group of 40–60 and 72.7% were in the age group of >60 years. Similarly in EBV as well as LMP1 the maximum positivity was seen in the age group of >40 years. Association of age with EBER, LMP-1 and EBV positivity was not found to be statistically significant in our study (p>0.05).
| Age | <20 years | 20 – 40 years | 40 – 60 years | >60 years | X2 | P- value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |||
| EBER | ||||||||||
| Present | 4 | 44.4 | 11 | 47.8 | 10 | 71.4 | 8 | 72.7 | 3.669 | 0.299 |
| Absent | 5 | 55.6 | 12 | 52.2 | 4 | 28.6 | 3 | 27.3 | ||
| EBV | ||||||||||
| Present | 6 | 66.7 | 14 | 60.9 | 12 | 85.70% | 9 | 81.8 | 1.884 | 0.595 |
| Absent | 3 | 33.3 | 9 | 39.1 | 2 | 14.3 | 2 | 18.2 | ||
| LMP-1 | ||||||||||
| Present | 5 | 55.6 | 14 | 60.9 | 9 | 64.3 | 9 | 81.8 | 3.367 | 0.338 |
| Absent | 4 | 44.4 | 9 | 39.1 | 5 | 35.7 | 2 | 18.2 | ||
Table 2: Distribution of age and EBER, LMP-1 and EBV positivity.
Association of gender with EBER, LMP 1 and EBV
Gender wise comparison was done in EBER, EBV and LMP1 (Table-3). In males, 61.5% has positive in EBER and 50% in females. But the difference was not found to be statistically significant (p=0.412). Similarly by applying chi square test in males, EBV was positive in 71.8% and in females it was 72.2%. Here also the difference was not significant. But in LMP 1, the males had 56.4% positivity and in females it was 83.3%. The difference between the gender in case of LMP1 is found to be statistically significant (p=0.037) (Table 3).
| Gender | Male | Female | X2 | P- value | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| EBER | ||||||
| Present | 24 | 61.5 | 9 | 50 | 0.673 | 0.412 |
| Absent | 15 | 38.5 | 9 | 50 | ||
| EBV | ||||||
| Present | 28 | 71.8 | 13 | 72.2 | 0.001 | 0.973 |
| Absent | 8 | 28.2 | 4 | 27.8 | ||
| LMP-1 | ||||||
| Present | 22 | 56.4 | 15 | 83.3 | 3.919 | 0.037 * |
| Absent | 17 | 43.6 | 3 | 16.7 | ||
Table 3: Association of gender with EBER, LMP-1 and EBV positivity.
Frequency of EBER, LMP 1 and EBV positive HL cases
All the 57 cases were histologically sub-typed, phenotyped& then studied for the presence of EBV (LMP 1, EBER and the EBV-combination of LMP1 & EBER) (Table 4). The results showed that 33/57(57.9%) cases are EBER positive and 37/57 cases (64.9%) are LMP-1 positive. Hence, IHC demonstrates EBV in approximately 71.9 % (41/57) of all HL cases (either EBER positive or LMP 1 positive or both), suggesting a role in tumorigenesis and the potential for EBV– targeted therapy.
| LMP 1 | EBV | EBER | ||||
|---|---|---|---|---|---|---|
|
N | %ge | N | %ge | N | %ge |
| Present | 37 | 64.9 | 41 | 71.9 | 33 | 57.9 |
| Absent | 20 | 35.1 | 16 | 28.1 | 24 | 42.1 |
Table 4: Frequency of EBER, LMP 1 and EBV positive HL cases.
Frequency of CD15 positivity in HL cases
Out of 57 cases, 55 were CD15 positive (96.5%) and 2 were negative (3.5%). All 57 cases were positive to CD30.
Association of HL Subtypes with EBER, LMP1 and EBV posivitiy
Association of HL subtypes with EBER, LMP1 and EBV positivity in given in Table 5. HL subtypes, EBER shows positivity more in MC (73.1%) and NS (52%). When we compare with respect to EBV, the positivity is more in MC (84.6%) and in NS (68%). In LMP1, it is maximum in NS (72%) compared to MC (65.4%). Individually EBV had significant result (0.041) and in LMP 1, there is no significant result (p=0.191) with the subtypes. But the combination of LMP1 and EBV has more significant with the subtypes compared to individual factor (p=0.034).
| HL subtypes | NS | MC | LR | LD | NLPHL | X2 | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | |||
| EBER | ||||||||||||
| Present | 13 | 52 | 19 | 73.1 | 0 | 0 | 1 | 100 | 0 | 0 | 10.314 | 0.034 * |
| Absent | 12 | 48 | 7 | 26.9 | 3 | 100 | 0 | 0 | 2 | 100 | ||
| EBV | ||||||||||||
| Present | 17 | 68 | 22 | 84.6 | 1 | 33.3 | 1 | 100 | 0 | 0 | 9.992 | 0.041 * |
| Absent | 8 | 32 | 4 | 15.4 | 2 | 66.7 | 0 | 0 | 2 | 100 | ||
| LMP-1 | ||||||||||||
| Present | 18 | 72 | 17 | 65.4 | 1 | 33.3 | 1 | 100 | 0 | 0 | 6.105 | 0.191 |
| Absent | 7 | 28 | 9 | 34.6 | 2 | 66.7 | 0 | 0 | 2 | 100 | ||
Table 5: Association of HL subtypes with with EBER, LMP-1 and EBV positivity.
Association of Ann Arbor Staging with EBER, LMP 1 and EBV
Stage wise comparison was done in all the three factors EBER, EBV and LMP 1 (Table 6). It was found that in EBER positivity was seen in 78.3% of stage III and 56.2% in Stage IV and the difference was found to be statistically significant. Similarly in EBV, it was more in Stage III and Stage IV. Positivity was found as 82.6 and 81.2% in these two stages respectively. But in stage I and Stage II also, nearly 50% positivity could be seen. So the difference of positivity between the different stages was not significant (p=0.099). Same result was seen in LMP1 also. Here the positivity can be seen in all the four stages. But still more in Stage III and Stage IV. But the difference was not to be significant (p=0.521) also.
| Ann-Arbor Stages | Stage I | Stage II | Stage III | Stage IV | X2 | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | No | % | |||
| EBER | ||||||||||
| Present | 0 | 0 | 6 | 42.9 | 18 | 78.3 | 9 | 56.2 | 10.73 | 0.013 * |
| Absent | 4 | 100 | 8 | 57.1 | 5 | 21.7 | 7 | 43.8 | ||
| EBV | ||||||||||
| Present | 2 | 50 | 7 | 50 | 19 | 82.6 | 13 | 81.2 | 6.275 | 0.099 |
| Absent | 2 | 50 | 7 | 50 | 4 | 17.4 | 3 | 18.8 | ||
| LMP-1 | ||||||||||
| Present | 2 | 50 | 6 | 42.9 | 14 | 60.9 | 11 | 68.8 | 2.258 | 0.521 |
| Absent | 2 | 50 | 8 | 57.1 | 9 | 39.1 | 5 | 31.2 | ||
Table 6: Association of Ann Arbor Staging with EBER, LMP 1 and EBV.
Discussion
In our study, HL showed bimodal age distribution with one peak (29.8%) in the age group of 20-29 years and another peak (14.0%) in the age group of 50-59 years. There were 39 males and 18 females with a male predominance in our study with male to female ratio 2.1:1
There was statistically significant association between gender and LMP1 (p=0.037) in our study, whereas Atif Ali Hashmi et al reported no significant association between gender and LMP1 expression [15]. There was no statistically significant association between gender and EBER (p=0.412). Overall, there was no statistically significant association between EBV and gender (p=0.973).
All the 57 cases were histologically sub-typed, phenotyped & then studied for the presence of LMP 1, EBER and the combination of these two (EBV=Either EBER positive or LMP 1 positive or both are positive). On morphological analysis, 26(45.6%) cases were classified as MC, 25(43.9%) cases were classified as NS, 03 (5.3%) cases were classified as LR, 01 (1.8%) case was classified as LD and NLPHL subtype was seen in 02 (3.5%) cases. 1/57 HL cases (1.8%) belong to LD subtype and is positive to LMP 1 (100%) which was in concordance with a study made by Muhammed Azhar et al. and Radha K et al. in which all the LD subtype cases were positive to LMP 1 [16,17]. In our study, out of 57 cases, 55 are CD15 positive, i.e., 96.5% of HL cases express CD 15. And all the 55 positive cases were CHL type and the other two CD15 negative cases were NLPHL type which was concordant with a study done by Pileri S et al. where in 80% of the HL patients were positive to CD15 [18].
In our study, maximum number of HL cases belong to Stage III (40.4%) followed by Stage IV (28.1%) and Stage II (25%). The least number of cases belong to Stage 1 (7%). It was similar to a study conducted by Cickusic E et al. in which maximum number of HL cases belong to Stage III (50%) followed by Stage IV (22.2%), II (22.2%) [9]. Among LMP1 positive patients, 68.8% Stage IV were LMP1 positive, followed by 60.9% Stage III, 42.9% Stage II and 50% of Stage I patients which did not show statistically significant association between LMP1 and Ann Arbor Staging.
Conclusion
Overall, we found among 57 cases of Hodgkin Lymphoma, 33 were EBER positive, 37 were LMP 1 positive and overall, 41 were EBV positive. There was statistically significant association between gender and LMP1 expression (p=0.037) in our study. Mixed Cellularity subtype being the most common histomorphological subtype followed by Nodular Sclerosis subtype in our study. There was a statistically significant association of EBER with morphological subtypes and Ann Arbor Staging. EBER expression was more frequently found in mixed cellularity subtype and in advanced stages of Hodgkin Lymphoma. There was no statistically significant association of LMP1 with morphological subtypes and Ann Arbor Staging. Individually, EBER expression had significant association (p=0.034) with HL subtypes and EBV also had significant association (p=0.041). With LMP1 expression alone, there was no significant association (p=0.191) with the subtypes, suggesting combination of EBER and LMP1 is has more significance compared than individual marker with HL subtypes. However, our study is limited by relatively low sample size. Although LMP1 expression is seen high in advanced stages which are similar to many other studies, it is also seen in early staged individuals. Hence, a final conclusion regarding LMP1 could not be established. Hence, further large scale study is required to analyse the impact of Epstein Barr Virus in Hodgkin Lymphoma etiology.
References
- Thacker N, Bakhshi S, Chinnaswamy G, Vora T, Prasad M, et al. (2017) Management of non-Hodgkin lymphoma: ICMR consensus document. The Indian Journal of Pediatrics 84: 382-392.
- Piccaluga PP, Agostinelli C, Gazzola A, Tripodo C, Bacci F, et al. (2011) Pathobiology of Hodgkin Lymphoma. Advances in Hematology 2011: 1-19.
- Flavell KJ, Murray PG (2000) Hodgkin’ s disease and the Epstein-Barr virus. J Clin Pathol: Mol Pathol 53: 262–269.
- Kang M, Kieff E (2015) Epstein – Barr virus latent genes. Experimental & Molecular Medicine 47: e131-16
- Roughan JE, Thorley-lawson DA (2009) The Intersection of Epstein-Barr Virus with the Germinal Center. J Virol 83: 3968–3976.
- Lerner MR, Andrews NC, Miller G, Steitz JA (1981) Two small RNAs encoded by Epstein–Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci USA 78:805 –809.
- Iwakiri D (2014) Epstein–Barr virus-encoded RNAs: key molecules in viral pathogenesis. Cancers 6: 1615–30.
- Margaret LG, Sally LG, Fiona EC, Michael B, Risa BM, et al. (2002) Guidelines for Interpreting EBER In Situ Hybridization and LMP1 Guidelines for Interpreting EBER In Situ Hybridization and LMP1 Immunohistochemical Tests for Detecting Epstein-Barr Virus in Hodgkin Lymphoma. Am J Clin Pathol 117: 259-67.
- Cickusić E, Mustedanagić-Mujanović J, Iljazović E, Karasalihović Z, Skaljić I (2007) Association of Hodgkin’s lymphoma with Epstein Barr virus infection. Bosn J Basic Med Sci 7: 58–65.
- Dinand V, Arya LS, Dawar R (2008) Unfavorable outcome in CD15 negative pediatric classical Hodgkin lymphoma. Pediatr Blood Cancer 50: 430.
- Tzankov A, Krugmann J, Fend F, Fischhofer M, Greil R, et al. (2003) The prognostic significance of CD20 expression in classical Hodgkin lymphoma: A clinico-pathological study of 119 cases. Clin Cancer Res 9: 1381–6
- Natkunam Y, Warnke RA, Montgomery K, Falini B, van de Rijn M (2001) Analysis of MUM1/IRF4 protein expression using tissue microarrays and immunohistochemistry. Modern Pathology 14: 686-94.
- Stein H, Marafioti T, Foss HD, Laumen H, Hummel M, et al. (2001) Down-regulation of BOB.1/OBF.1 and Oct2 in classical Hodgkin disease but not in lymphocyte predominant Hodgkin disease correlates with immunoglobulin transcription. Blood 97: 496–501
- Madanagopaal LR, Subashchandrabose P, Sivanandam S (2016) Analysis of PAX5 expression of Hodgkin ’ s and non- Hodgkin ’ s lymphomas : in comparison with routine panel. Archives of Cytology and Histopathology Research 1: 4–13.
- Atif AH, Zubaida FH, Kashif AH, Muhammad IZ, Muhammad ME et al. (2017) Latent membrane protein LMP-1 expression in HL and its correlation with clinical and histological parameters. World Journal of Surgical Oncology 15: 89
- Muhammad A, Hafeez ud Din, Iqbal M, Shoaib NH, Farhan A, et al. (2016) Frequency of EBV in Classical Hodgkin Lymphoma. J Ayub Med Coll Abbottabad 28: 271-215.
- Radha K, Shanthi P, Madhavan M, Senthamarai A (1997) Study of association of Epstein-Barr virus with Hodgkin’s disease. Ind J Pathol Microbiol 40: 351-4.
- Pileri S, Sabattini E, Tazzari PL, Gherlinzoni F, Zucchini L, et al. (1991) Hodgkin's disease: Update of findings. Haematologica 76: 175-82.