Research Article, Biomater Med Appl Vol: 2 Issue: 1
Collagen Based Multilayer Scaffolds for Meniscus Tissue Engineering: In Vivo Test Results
Albana Ndreu Halili1,2,3, Siyami Karahan4, Baris Kürüm4 and Vasif Hasirci1,2,5*
1METU, Department of Biotechnology, Ankara, Turkey
2BIOMATEN, METU Center of Excellence in Biomaterials and Tissue Engineering, Ankara, Turkey
3Aleksander Moisiu University, Department of Information Technology, Durres, Albania
4Kırıkkale University, Faculty of Veterinary Medicine, Kırıkkale, Turkey
5METU, Department of Biological Sciences, Ankara, Turkey
*Corresponding Author : Prof. V. Hasirci
Department of Biological Sciences, METU 06800 Ankara, Turkey
E-mail: vhasirci@metu.edu.tr
Received: January 01, 2018 Accepted: January 17, 2018 Published: January 23, 2018
Citation: Halili AN, Kürüm B, Karahan S, Hasirci V (2018) Collagen Based Multilayer Scaffolds for Meniscus Tissue Engineering: In Vivo Test Results. Biomater Med Appl 2:1. doi: 10.4172/2577-0268.1000112
Abstract
Meniscus is an important component of the knee joint since it performs several crucial functions such as shock absorption, load bearing and transmission, maintenance of joint stability, and lubrication. The results of common meniscal injury repair approaches are not fully satisfactory with low mechanical properties and long regeneration times. A 3D collagen-based construct consisting of multilayers of lyophilized sponges separated by electrospun fibrous mats was prepared previously to serve as a substitute for meniscus. Mechanical properties of the construct were studied in vitro and a 3 to 4 fold increase was observed when a double crosslinking method was used.
Rabbit meniscal cells were cultured in vitro, expanded and seeded onto the polymer scaffolds. 2 weeks later the substitute was implanted to the medial compartment of the rabbit knee joint. The implants were studied 3 and 10 weeks after transplantation. Histological and microscopical characterization showed a significant difference between the groups (Group I: control; Group II: cell free substitute and Group III: cell seeded substitute) with Week3 sample scores. Group III healing score was significantly lower than I and II, which was probably due to the the fibrous tissue surrounding the cell seeded material but this resulted in lower immunological responses. Moreover, the scores decreased from Week3 to Week10 indicating healing. Even though there were no statistically significant differences, the lowest values were observed with the tissue engineered substitute. Therefore, it can be concluded that in vivo studies showed the potential of the cell seeded artificial meniscus.
Keywords: In vivo studies; Meniscus; Multilayered 3D scaffold; Tissue engineering
Introduction
Meniscus is an important component of the knee joint since it performs several crucial functions such as shock absorption, load bearing and transmission, maintenance of joint stability, and lubrication. Meniscal tears are the most common intra-articular injuries in the knee. Spontaneous healing is expected to occur in the peripheral zone-related tears due to the presence of blood supply, however, when the inner parts of the meniscus are damaged the healing capability by itself is almost none or very limited [1-8] have shown that the type of tear also influences the degree of healing [9].
A variety of repair methods have been developed to increase the level of healing. Treatments can be nonsurgical and surgical. Nonsurgical treatments include application of certain solutions inside the joint like addition of synovial fluid or chondroitin sulphate-hyaluronic acid combinations. The surgical methods include procedures such as meniscal repair, meniscectomy, meniscus replacement and tissue engineering.
Meniscus repair is mostly used to solve the problem only when the tears are not so complex and the meniscal tissues are not degenerated to a great extent [10]. On the other hand, meniscectomy is the surgical removal of all or part of an injured meniscus and can be classified respectively as total meniscectomy and partial meniscectomy [11]. It has been shown that both total and partial meniscectomy (to a lesser degree) lead to osteoarthritic degeneration of the knee joint due to abnormal stresses applied on the articular cartilage, but it still remains an option in case of irreparable tears [3,5,12,13]. Moreover, post-treatment studies prove that meniscectomy increases the risk of radiographic degenerative changes, associated with severe pain and dysfunction, which is expected to progress over a long-term period resulting in decreased patient satisfaction [14,15].
A third method used to treat meniscus injuries is to replace the damaged tissue with either a natural or synthetic materials. Meniscal allografts, prostheses or substitutes are the three main approaches utilized to date. Results obtained from meniscal allografts seem to be promising but there are no long term results yet. Moreover, there are certain constraints like paucity of donor tissue, complexity of the surgery, risk of disease transmission, and the occurrence of articular degeneration after transplantation, which limit meniscal transplantations [16,17].
Due to the above limitations of current surgical treatments, there is a need for a more consistent approach to restore the functionalities of the injured meniscus, which resulted in the development of scaffold-based techniques [18]. Four types of materials are used to prepare meniscal scaffolds. They are tissue-derived materials [1,19-21], extracellular matrix (ECM) components [22,23], synthetic polymers (such as polyurethane (PU), polyglycolic acid (PGA), poly(lactic acid-co-glycolic acid) (PLGA) and poly(ε-caprolactone) (PCL)) and hydrogels. Comparing all the above four types of scaffolds, none of them has been shown to be superior to the others in terms of biological and biomechanical properties. A possible solution is to combine multiple materials and develop hybrid materials or structure with enhanced properties that facilitate easily tissue regeneration [18,23].
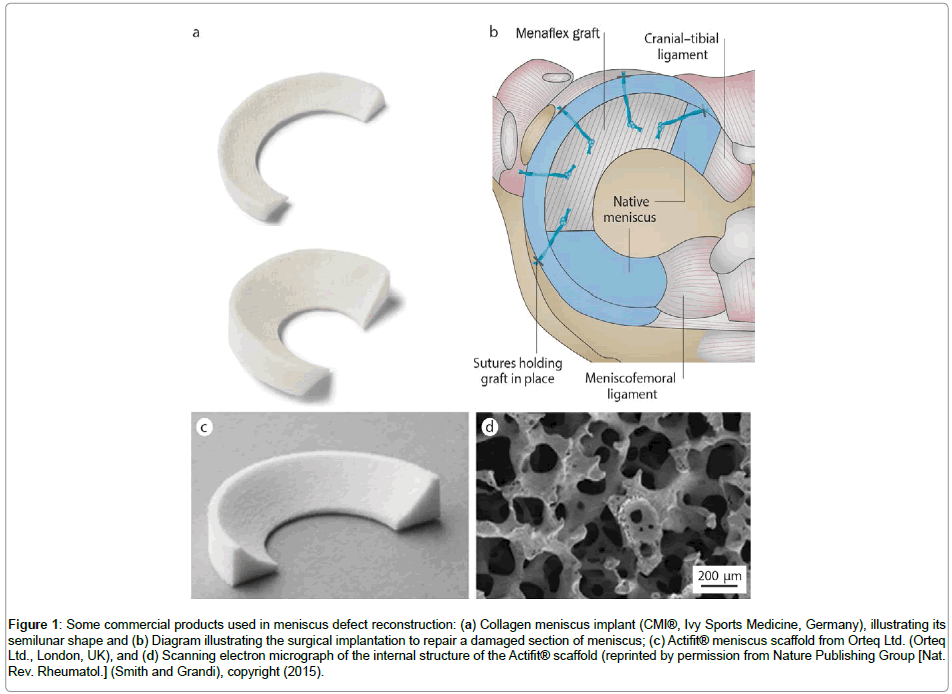
Nowadays, there are three types of biodegradable and biocompatible scaffolds on the market which can reconstruct the segmental meniscus defects, namely Menaflex CMI from ReGen Biologics, Inc., Actifit® scaffold from Orteq Ltd. (Figure 1), and NUsurface® Meniscus Implant from Active Implants. While the first two are partial meniscal substitutes with equivalents in histological, radiological, and clinical evaluations [24,25], the latter is the first total meniscal (medial) substitute, and has been used in Europe under CE Mark since 2008 and in Israel since 2011 [26].
Figure 1: Some commercial products used in meniscus defect reconstruction: (a) Collagen meniscus implant (CMI®, Ivy Sports Medicine, Germany), illustrating its semilunar shape and (b) Diagram illustrating the surgical implantation to repair a damaged section of meniscus; (c) Actifit® meniscus scaffold from Orteq Ltd. (Orteq Ltd., London, UK), and (d) Scanning electron micrograph of the internal structure of the Actifit® scaffold (reprinted by permission from Nature Publishing Group [Nat. Rev. Rheumatol.] (Smith and Grandi), copyright (2015).
Menaflex CMI and Actifit® scaffolds have received the Conformité Européenne (CE) mark in Europe, and NUsurface® Meniscus Implant has been used in Europe under CE Mark since 2008 and since 2011 in Israel. The NUsurface® Meniscus Implant is made from polycarbonateurethane (PCU)– a medical grade plastic. As a result of its unique materials and composite structure and design, it does not require fixation to bone or soft tissues. Its clinical results in the short term was promising. According to the published data, about 130 middle aged patients were treated, and a significant pain relief was reported 12 months post-operation [27]. However, it has to be mentioned that although these products are designed to mimic the function of the natural meniscus or stimulate the growth of new tissues, they still needed various improvements in their structure and material design [18,28].
Ideally, a meniscus implant or transplant should mimic the size, shape, vascularity and biomechanical properties of a natural meniscus. Tissue engineering of knee meniscus could be a viable alternative for the treatment of meniscal complications. A large number of studies were carried out in vitro and in vivo [29-31] but to date no product could satisfactorily mimic the shape, structure and mechanical properties of an autograft, and meet the load-distributing demands of the knee [32-34].
We designed a collagen-based, tissue engineered meniscus substitute. The main approach was to prepare a construct using a natural biocompatible material which would have optimum mechanical properties under tensile, compressive and shear forces. The construct consisted of three different collagen based foams with varying mechanical properties stacked on top of each other creating a multilayer 3D structure. In order to provide a hydrogel layer as in the case of the native tissue, the upper layer was made of a combination of natural polysaccharides chondroitin sulfate-hyaluronic acid-collagen (Coll-CS-HA). The central and lower layers were composed of collagen type I which were prepared to have decreasing porosity and therefore increasing mechanical properties. In addition, in order to improve the mechanical properties and mimic to a some extent the fibrous nature of the native meniscus, electrospun collagen-poly(lactic acid-coglycolic acid) (coll-PLGA) mats with fibers unaxiaxially aligned were introduced to the 3D meniscus construct [13,23].
After obtaining promising in vitro results [23], the 3D construct was implanted into the medial compartment of the New Zealand rabbit knee joints. In this study, the in vivo results of this tissue engineered 3D meniscus substitute are being presented.
Materials and Methods
Materials
Collagen type I from bovine Achilles’ tendon (BACT I), chondroitin sulfate A (CS) sodium salt from bovine trachea, hyaluronic acid (HA) potassium salt from human umbilical cord, amphotericin B, Trypsin from bovine pancreas were from Sigma-Aldrich (USA). Penicillin/Streptomycin was from Fluka (Switzerland) and Trypsin– EDTA (0.25%) was bought from HyClone, Thermo Scientific (USA). Poly(L-lactic acid-co-glycolic acid), (PLGA50:50, Resomer-RG503H) was supplied by Boehringer-Ingelheim (Germany) and Appli-Chem (USA). Cell medium DMEM/F12 (1:1) and Trypan blue were obtained from GIBCO Invitrogen Inc. (USA). OsteoDec was purchased from Bio Optica (Italy). The histological stains including Toludine blue, hematoxylin and eosin were obtained from Sigma.
Methods
Preparation of 3D samples for in vivo application: The 3D construct design was based on stacking of 3 dehydrothermally (DHT) crosslinked collagen-based foams. The top layer was composed of freeze dried foam containing Bovine Achilles Tendon Collagen (BATC), chondroitin sulfate (CS) and hyaluronic acid (HA). On the other hand, the middle and bottom layers were pure BATC collagen foams prepared by freezing at different temperatures to obtain foams with varying pore sizes and mechanical strengths. Electrospun mats of Coll-PLGA fibers were placed in between the foams and then all were glued together with collagen solution (15%, w/v). Details about the stacking procedure were described before [23]. The 3D samples with dimensions appropriate for rabbits (length: 10 mm, width: 2-2.5 mm) were prepared in triplicates for each in vivo application.
Isolation and culture of rabbit meniscal cells: Meniscus tissue was totally removed from New Zealand rabbits and the cells were isolated as we reported earlier [23]. The isolated meniscus cells were seeded and cultured into 75 cm2 tissue culture flasks until a sufficient number of cells was obtained. Cell culture medium used was DMEM/ F12 (1:1) containing 10 %, v/v fetal bovine serum, 1 % antibiotics (Penicillin–Streptomycin) and 1 μL/mL amphotericin B and the cells were passaged until Passage 3.
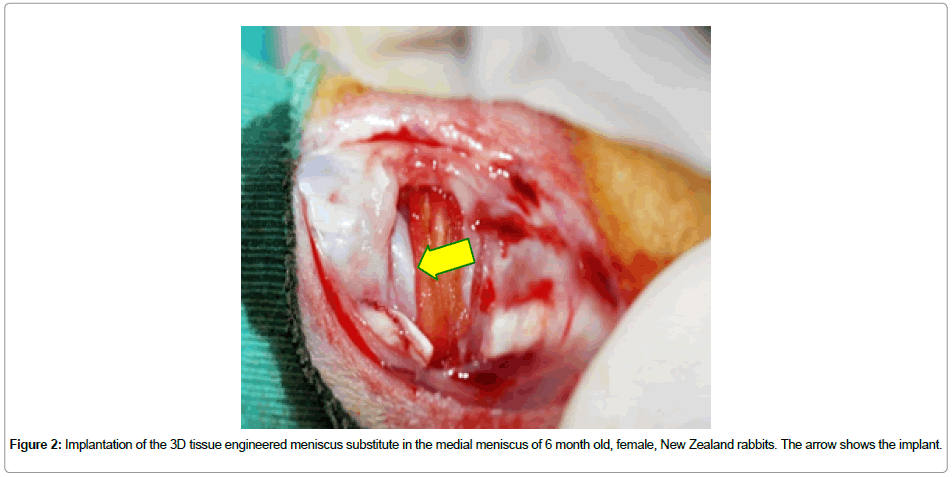
Cell seeding onto collagen-based scaffolds: For cell seeding, the rabbit meniscal cells were detached from the tissue culture flask surfaces with Trypsin (0.25 %)-EDTA (2 min at 37°C). Excess Trypsin was deactivated with serum and the cells were collected by centrifugation (3,000 rpm, 5 min). The cell pellet was resuspended in 3 mL fresh medium. After that, the cells were stained with Trypan blue and live cells were counted with a hemocytometer. Fibrochondrocyte suspension (50 μL) with a cell density of 106 cells/cm2 was seeded on the 3D constructs. The scaffolds were incubated in the CO2 incubatorfor 2 h in order to allow attachment of the cells. Finally, 1 mL of medium was added into each well and the cells were maintained in the carbon dioxide incubator with a daily medium change. After 10 days of culture the meniscus shaped 3D structure was implanted into the knees (medial meniscus) of New Zealand rabbits. In total, 42 (6 mo old female) rabbits were used to complete the experiments. 14 of the rabbits were used as control (the medial meniscus was completely removed) (n = 7) for 2 time points. On the other hand, the medial menisci of the remaining 28 animals were replaced with the tissue engineered construct. 14 knees were replaced with unseeded artificial meniscus (n=7) whereas the other 14 were replaced with cell-seeded menisci (n=7). The animals were sacrificed at the end of Week 3 and 10 of implantation, respectively, and their medial menisci were removed (resected) to be studied both microscopically and histologically (Figure 2).
In vivo studies: The samples used for in vivo experimentation were implanted in the following sequence after approval by the Kırıkkale University Ethical Committee for Animal Experiments (Document No: 09/16)
Group I: control group. Medial meniscus was completely removed and was not replaced with any substitute.
An incision was made medially to the left knee joint of the rabbits of this group by cutting the medial collateral ligament. Then, the medial meniscus was completely removed from the joint by cutting its connections to menisco-tibial ligaments (cranial and caudal). After that, the joint was closed (using routine procedure) by suturing the medial collateral ligament to the medial. Rabbits were sacrificed after Week3 (n = 7) and Week10 (n = 7).
Group II: Medial meniscus was completely removed and unseeded collagen-based meniscus substitute (10 mm long and 2-2.5 mm wide) was implanted in the gap.
An incision was made medially to the left knee joint of the rabbits by cutting the medial collateral ligament. The medial meniscus was completely removed from the joint by cutting its connections to menisco-tibial ligaments (cranial and caudal). After that, the unseeded collagen-based meniscus substitute was implanted into the gap left behind by the removed tissue by attaching it to the synovial membrane. Finally, the joint was closed by suturing the collateral ligamentum to the medial. Rabbits were sacrificed at the end of Week3 (n = 7) and Week10 (n = 7).
Group III: Medial meniscus was completely removed and rabbit MSC seeded collagen meniscus substitute (10 mm long and 2-2.5 mm wide) was implanted in the gap.
The implantation procedure was as described for the unseeded artificial meniscus substitute.
After the animals were sacrificed, the knee joints were removed and kept in 10% formalin for 48 h. This was followed by a decalcification process in Osteodec decalcifying solution. Then, the decalcified joints underwent routine dehydration procedures and were embedded in paraffin blocks. Finally, the knee joints in paraffin blocks were cut at a thickness of 7μm on a rotary microtome. The sections were then stained with Hematoxylin-Eosin and Toluidin blue, and examined under a microscope (Leica DM5000B).
Statistical analysis and the modified scoring system: A modified scoring system based on the observations of the study and previous studies was used as given in Table 1 [35,36]. The scores calculated were compared by SAS program by means of 2-way ANOVA analysis and the reason for the difference among the groups was explained with LSD.
| Score | Observation |
|---|---|
| 0 | Fibrocartilage and some fibrous tissue presence |
| 1 | A tight fibrous structure (mostly matrix) |
| 2 | Mostly a fibrous structure (with many cells) and a low presence of inflammatory cell infiltration |
| 3 | A loose connective tissue and inflammatory cell infiltration |
| 4 | Intensive inflammatory regions |
Table 1: Scoring system used in the evaluation of tissue response.
Results and Discussion
A 3D collagen based scaffold was designed and tested in vitro in our previous study [23]. Porosity was one of the parameters that were considered to ensure cell migration toward the inner parts of the scaffold. As expected, the highest porosity and pore size distribution was observed with Coll-CS-HA foams compared to pure collagen ones (prepared at -20°C and -80°C). Concerning the 3D construct, since it is a combination of the three foam layers, a broader distribution of pores was obtained with an increase in the fraction of large pores (100- 200 μm) due to the presence of the highly porous Coll-CS-HA foam and also of middle-sized pores as a result of -20°C and -80°C collagen foams [35]. These properties appear to be suitable for meniscus tissue engineering applications.
Regarding the mechanical properties, Coll-CS-HA showed the lowest mechanical properties and the highest compressive modulus was obtained with double crosslinked -80°C foams. As was expected, the highest compressive and tensile properties were obtained with the 3D construct. In the dry state the 3D construct showed higher compressive and shear properties than the natural tissue but the tensile properties were much lower (Table 2).
| Coll-CS-HA (UL) | -20°C Coll (ML) | -80°C Coll (LL) | 3D construct | |
|---|---|---|---|---|
| Degradation | ||||
| 50% | 73% | 73% | 38% | |
| Mechanical Properties (in dry state) | ||||
| Compressive Modulus (kPa) X | 118.3 ± 4.9 | 228.3 ± 30.9 | 234.9 ± 16.2 | 444.6 ± 89.6 |
| Young’s Modulus (MPa) X | 1.2 ± 0.4 | 2.1 ± 0.2 | 1.3 ± 0.4 | 2.9 ± 0.5 |
| Shear Modulus (kPa) X | 160.3 ± 19.6 | 186.5 ± 33.8 | 138.2 ± 21.1 | 194.3 ± 20.3 |
X: Native meniscus values: Compressive Modulus = 150 kPa; Young’s Modulus = 100-300 MPa; Shear Modulus = 120 kPa
Table 2: Degradation and mechanical properties of the 3D construct in comparison with the individual collagen-based foams (adapted from Ndreu Halili A., 2011).
All the individual foams and the 3D construct were then seeded with human fibrochondrocytes (initial cell density 1x105 cells/sample) for assessment of their suitability to serve as a meniscus substitute and the highest cell proliferation was obtained with the 3D constructs followed by Coll-CS-HA foams and then -20°C and -80°C collagen foams. The cells kept increasing in number very satisfactorily in the 3D construct. Moreover, unconfined compression test was performed on all collagen-based foams and the 3D construct after 1, 21 and 45 days of cell culture and they were compared with the unseeded control scaffolds to study the effects of cell presence on the mechanical properties of the tissue. Even after one day of culture, the cells present in the scaffolds slightly increased the compressive properties of the scaffold. A decline was observed on Day21 of culture for all scaffolds (except the 3D construct) and this was interpreted to be due to the degradation of the scaffold. Later, the values increased significantly and cells deposited their own GAGs and collagen which had a significant role in increasing the compressive properties of the scaffold [13] (Table 3).
| Coll-CS-HA (UL) | -20ºC Coll (ML) | -80ºC Coll (LL) | 3D construct | |||||
|---|---|---|---|---|---|---|---|---|
| Cell Proliferation (Day21) | ||||||||
| Cell Number (x105) | 3.75 | 1.83 | 2.83 | 3.90 | ||||
| Compressive Modulus (kPa) | Day1 (unseeded) | 0.5 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.1 | 4.1 ± 0.1 | |||
| Day1 (seeded) | 0.9 ± 0.5 | 2.9 ± 1.1 | 3.4 ± 0.2 | 4.3 ± 0.4 | ||||
| Day45 (seeded) | 1.2 ± 0.1 | 5.1 ± 0.4 | 6.1 ± 1.2 | 12.1 ± 1.9 | ||||
Table 3: Cell proliferation results and compressive properties of collagen-based scaffolds (adapted from Ndreu Halili A., 2011).
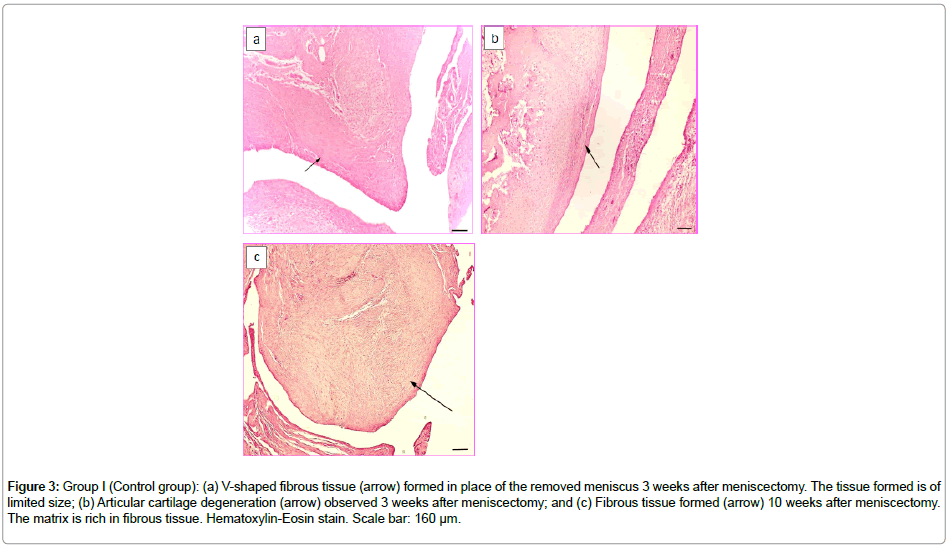
After successful in vitro results, the 3D construct was then assessed in this study under in vivo conditions by implanting in the medial compartment of New Zealand rabbit knee joints. The GroupI (control group, with no construct) knees were studied on Week3 and Week10 after total meniscectomy. On Week3, a fibrous tissue originating from the synovium site, possessing excessively high proliferative properties was observed (Figure 3a). Certain regions of this fibrous tissue resembled the classical loose connective tissue while some other regions appeared as a fibrous tissue mostly composed of young fibroblastic cells. In addition, an early degeneration of the articular cartilage was noteworthy in this case due to the meniscectomy performed (Figure 3b). Toluidine blue staining was not observed inthe synoviumorigin fibrous tissue indicating presence of no glycosaminoglycan (GAG) deposition.
Figure 3: Group I (Control group): (a) V-shaped fibrous tissue (arrow) formed in place of the removed meniscus 3 weeks after meniscectomy. The tissue formed is of limited size; (b) Articular cartilage degeneration (arrow) observed 3 weeks after meniscectomy; and (c) Fibrous tissue formed (arrow) 10 weeks after meniscectomy. The matrix is rich in fibrous tissue. Hematoxylin-Eosin stain. Scale bar: 160 μm.
Week10 samples also showed a fibrous tissue originating from the synovium and replacing the removed natural meniscus. The tissue formed was meniscus-like in shape, and moreover, it histologically resembled an irregular, dense connective tissue (Figure 3c). There was also an increase in the knee joint articular cartilage degeneration.
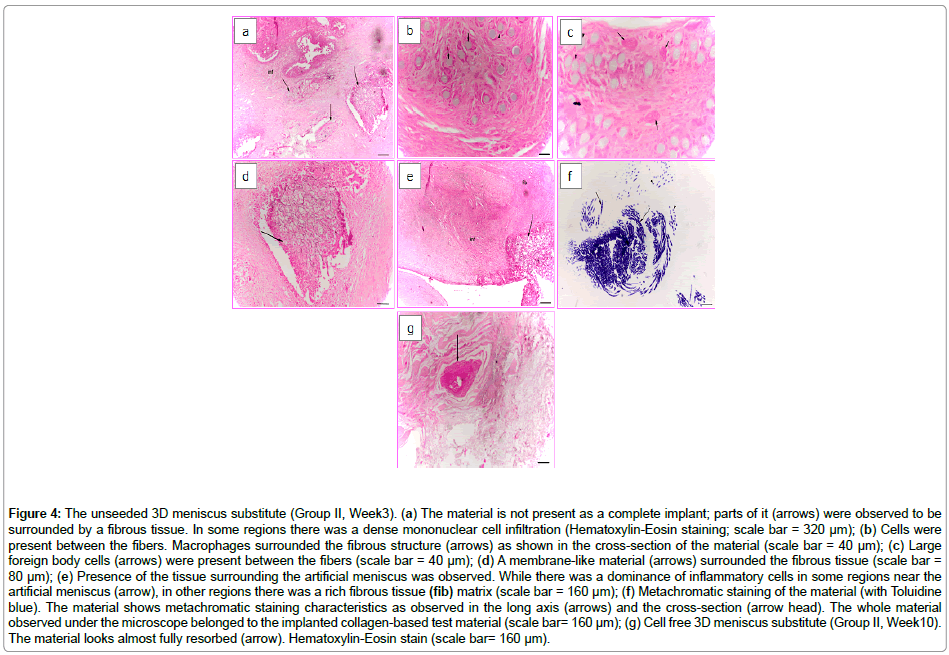
The Group II animals consisted of rabbits whose meniscus were replaced totally with unseeded collagen based 3D construct. The H&E stain showed that the implanted material was trapped inside the synovium-associated fibrous tissue (Week3); however, the material was not stable as a whole structure, probably due to the degradation of collagen in time (Figure 4a). Moreover, foreign body cells, macrophages and fibroblasts were observed in between the fibrous material (Figure 4b and 4c). Cell infiltration from the native tissue towards the pores of the material (Figure 4d) was seen and also dense mononuclear cell infiltration near the material, and inside and around the connective tissue which surrounded the material was observed. On the other hand, there was a fibrous tissue development in regions where there was no infiltration of cells (Figure 4e). Figure 4f shows the fibrous structure of the material which was stained with Toluidine blue; however, no stained cells were observed around them. The infiltration of the scaffold is observed.
Figure 4: The unseeded 3D meniscus substitute (Group II, Week3). (a) The material is not present as a complete implant; parts of it (arrows) were observed to be surrounded by a fibrous tissue. In some regions there was a dense mononuclear cell infiltration (Hematoxylin-Eosin staining; scale bar = 320 μm); (b) Cells were present between the fibers. Macrophages surrounded the fibrous structure (arrows) as shown in the cross-section of the material (scale bar = 40 μm); (c) Large foreign body cells (arrows) were present between the fibers (scale bar = 40 μm); (d) A membrane-like material (arrows) surrounded the fibrous tissue (scale bar = 80 μm); (e) Presence of the tissue surrounding the artificial meniscus was observed. While there was a dominance of inflammatory cells in some regions near the artificial meniscus (arrow), in other regions there was a rich fibrous tissue (fib) matrix (scale bar = 160 μm); (f) Metachromatic staining of the material (with Toluidine blue). The material shows metachromatic staining characteristics as observed in the long axis (arrows) and the cross-section (arrow head). The whole material observed under the microscope belonged to the implanted collagen-based test material (scale bar= 160 μm); (g) Cell free 3D meniscus substitute (Group II, Week10). The material looks almost fully resorbed (arrow). Hematoxylin-Eosin stain (scale bar= 160 μm).
After 10 weeks of implantation, the collagen-based unseeded material was almost totally resorbed (Figure 4g). The remaining material was surrounded by the tissue and reduced in size due to resorption. The presence of macrophages and large foreign body cells in the center and around the material was observed.
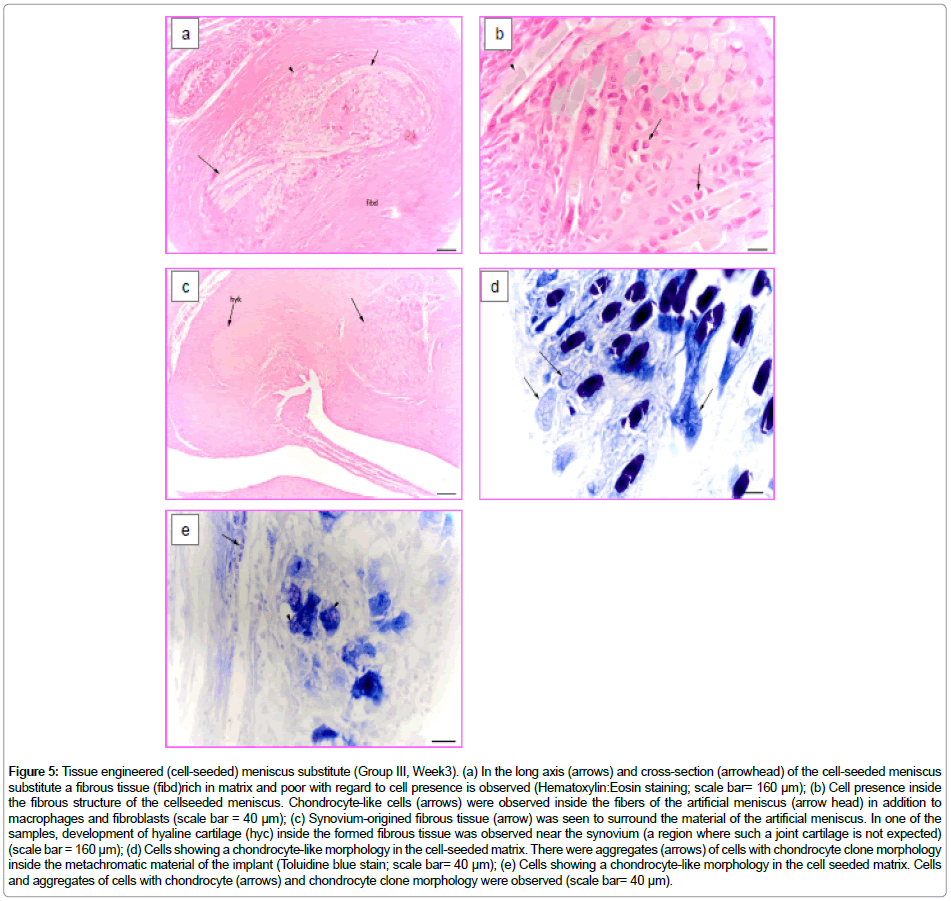
After 3 weeks of implantation of the tissue engineered (cell seeded) collagen-based meniscus of Group III, the material was trapped inside the synovium-origined fibrous tissue. Similar to Group II samples, the degradation of the scaffold was observed. The invading material was seen to be separated by the fibrous tissue (Figure 5a). In this case foreign body giant cells, macrophages and fibroblasts were seenless frequently between the fibrous material. It is important to state that chondrocyte-like cells could be observed inside the fibrous material (Figure 5b).
Figure 5: Tissue engineered (cell-seeded) meniscus substitute (Group III, Week3). (a) In the long axis (arrows) and cross-section (arrowhead) of the cell-seeded meniscus substitute a fibrous tissue (fibd)rich in matrix and poor with regard to cell presence is observed (Hematoxylin:Eosin staining; scale bar= 160 μm); (b) Cell presence inside the fibrous structure of the cellseeded meniscus. Chondrocyte-like cells (arrows) were observed inside the fibers of the artificial meniscus (arrow head) in addition to macrophages and fibroblasts (scale bar = 40 μm); (c) Synovium-origined fibrous tissue (arrow) was seen to surround the material of the artificial meniscus. In one of the samples, development of hyaline cartilage (hyc) inside the formed fibrous tissue was observed near the synovium (a region where such a joint cartilage is not expected) (scale bar = 160 μm); (d) Cells showing a chondrocyte-like morphology in the cell-seeded matrix. There were aggregates (arrows) of cells with chondrocyte clone morphology inside the metachromatic material of the implant (Toluidine blue stain; scale bar= 40 μm); (e) Cells showing a chondrocyte-like morphology in the cell seeded matrix. Cells and aggregates of cells with chondrocyte (arrows) and chondrocyte clone morphology were observed (scale bar= 40 μm).
At the same time, less mononuclear cellular infiltration was seen inside the tissue near and around the implanted material. This fibrous tissue showed dense connective tissue characteristics. In one of the samples a locally developed hyaline cartilage tissue was observed (Figure 5c). Some cell aggregates resembling chondrocytic clones were seen in the implanted material (both fibers and foams) (Figure 5d and 5e). Moreover, cells showing metachromatic properties, which are probably chondrocytes, were detected.
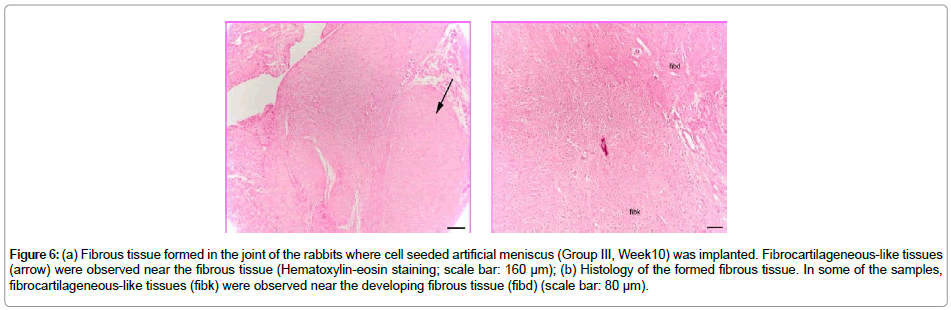
At the end of 10 weeks of implantation the implanted Group III material was further reduced in size and almost totally resorbed by the synovium origined fibrous tissue. There were macrophages and giant cells in the center and around the resorbed material. The resorbed regions showed similar characteristics with the unseededmeniscussubstitute. In some of the samples, chondrocytelike cells were observed in the tissue near the empty joint region related to synovium. There were regions resembling the fibrocartilagenous structure (Figure 6a and 6b).
Figure 6: (a) Fibrous tissue formed in the joint of the rabbits where cell seeded artificial meniscus (Group III, Week10) was implanted. Fibrocartilageneous-like tissues (arrow) were observed near the fibrous tissue (Hematoxylin-eosin staining; scale bar: 160 μm); (b) Histology of the formed fibrous tissue. In some of the samples, fibrocartilageneous-like tissues (fibk) were observed near the developing fibrous tissue (fibd) (scale bar: 80 μm).
Assessment Scores of the 3 Test Groups
The average scores calculated were 4.43 and 3.15 for Group I-Week3 and Week10 samples, respectively, and the difference was statistically significant (p<0.004). For Group II samples on Week3 the score was 4.57 and on Week 10 it was 3.50 and this decrease was statistically significant (p<0.02). For Group III on the other hand the average score for Week3 samples of cellseeded meniscus substitute was 3.43, and on Week10 it was 3.0; however, this decrease was not statistically significant (p>0.05) (Table 4).
| Sample | Scores | |
|---|---|---|
| Week 3 | Week 10 | |
| Group I Control, empty | 4.43 ± 0.53 | 3.14 ± 0.69 |
| Group II Unseeded | 4.57 ± 0.53 | 3.45 ± 1.05 |
| Group III Cellseeded | 3.43 ± 0.77* | 3.00 ± 0.89 |
Table 4: Scores for the test groups implanted with different meniscal substitutes. After 3 and 10 weeks of surgery.
The overall assesment of the scores show that inflammatory response is lowest in the meniscal cell loaded tissue engineered substitutes (Group III) and highest in the cell free implant (Group I). Another observation is that the scores decreased with time (from 3 weeks to 10 weeks) indicating healing.
Conclusion
A multilayer, 3D construct was prepared from collagen to serve as in the repair of meniscus defects. The construct was implanted into the medial compartment of the rabbit knee joint. Histological and microscopical characterization showed no excessive inflammatory response in any of the groups consisting of empty control (Group I), cell-free substitute (Group II) and rabbit fibrochondrocyte seeded substitute (Group III) and the most promising results were obtained with the tissue engineered Group III scaffolds.
A significant difference between groups was observed with Week3 samples. There was no significant difference between Group I (4.43) and Group II (4.57). The small difference was probably due to the immunologic response to Group II sample that was caused by the presence of the biomaterial. However, Group III score (3.43) was significantly lower than those of Groups I (p<0.05) and II (p<0.01) (Table 3). This difference was probably due to the fibrous tissue that surrounded the cell seeded material (Group III) resulting in lower immunological responses. Week10 results were lower than the Week3 samples, and even though there were no statistically significant differences between the samples, the lowest values were still observed with the tissue engineered Group III substitute.
As a result, it can be concluded that in vivo studies showed the potential of the artificial meniscus. However, some further testing should be made using larger animal models (like sheep or goat) that possess bigger menisci and have anatomy more similar to the human than the rabbits.
Acknowledgements
We acknowledge the financial support by the Ministry of Industry and Commerce of Turkey through the SanTez project (00356.STZ.2009-1) and the collaboration with Ars Arthro Biotech. Co. (Ankara, Turkey). We are grateful to METU through its support with BAP projects. We are also grateful to TUBITAK (Program no: 2215) for the Ph.D. grant to A.N.H.
Conflict of Interest Statement
The authors declare that there is no conflict of interest
References
- Sweigart MA, Athanasiou KA (2001) Toward tissue engineering of the knee meniscus. Tissue Eng 7: 111-129.
- Buma P, Ramrattan NN, van Tienen TG, Veth Rene PH (2004) Tissue engineering of the meniscus. Biomaterials, 25: 1523-1532.
- McDermott ID (2006) Mini-symposium: soft tissue knee problems: (ii) Meniscal tears. Current Orthopaedics 20: 85-94.
- Hoben GM, Athanasiou KA (2006) Meniscus repair with fibrocartilage engineering. Sports Medicine Arthroscopy 14: 129-137.
- Schoenfeld AJ, Landis WJ, Kay DB (2007) Tissue-engineered meniscal constructs. Am. J Orthop 36: 614-620.
- Van der Bracht H, Verdonk R, Verbruggen G, Elewaut D, Verdonk P (2007) Cell-based meniscus tissue engineering, in: Topics in tissue engineering 3.
- Drengk A, Stürmer KM, Frosch KH (2008) Current concepts in meniscus tissue engineering. Curr Rheumatol Rev 4: 196-201.
- Angele P, Johnstone B, Kujat R, Zellner J, Nerlich M, et al. (2008) Stem cell based tissue engineering for meniscus repair. J Biomed Mater Res A 85: 445-455.
- Newman AP, Anderson DR, Daniel AU, Dales MC (1989) Mechanics of the healed meniscus in a canine model. Am J Sports Med 17: 164-175.
- Arnoczky SP, DiCarlo EF, O’Brien SJ, Warren RF (1992) Cellular repopulation of deep-frozen meniscal autografts: an experimental study in the dog. Arthroscopy 8: 428-436.
- Jeong HJ, Lee SH, Ko CH (2012) Meniscectomy. Knee Surg Relat Res 24: 129-136.
- Van Tienen TG, Hannink G, Buma P (2009) Meniscus replacement using synthetic materials. Clin Sports Med 28: 143-156.
- Ndreu Halili A (2011) Collagen-based meniscus tissue engineering: Design and application (PhD thesis), Middle East Technical University.
- Johnson RJ, Kettelkamp DB, Clark W, Leaverton P (1974) Factors affecting late results after meniscectomy. J Bone Jt Surg Am 56: 719-729.
- Roos EM, Östenberg A, Roos H, Ekdahl C, Lohmander LS (2001) Long-term outcome of meniscectomy: Symptoms, function, and performance tests in patients with or without radiographic osteoarthritis compared to matched controls. Osteoarthr Cartil 9: 316-324.
- Crook TB, Ardolino A, Williams LAP, Barlow IW (2009) Meniscal allograft transplantation: A review of the current literature. Ann. R Coll Surg Engl 91: 361-365.
- Lee SR, Kim JG, Nam SW (2012) The tips and pitfalls of meniscus allograft transplantation. Knee Surg. Relat Res 24: 137-145.
- Sun J, Vijayavenkataraman S, Liu H (2017) An Overview of Scaffold Design and Fabrication Technology for Engineered Knee Meniscus. Materials 10: 29-48.
- Cook JL, Tomlinson JL, Kreeger JM, Cook CR (1999) Induction of meniscal regeneration in dogs using a novel biomaterial. Am J Sports Med 27: 658-665.
- Stapleton TW, Ingram J, Katta J, Knight R, Korossis S, et al. (2008) Development and characterization of an acellular porcine medial meniscus for use in tissue engineering. Tissue Eng Part A 14: 505-518.
- Shalumon K, Anulekha K, Chennazhi K, Tamura H, Nair S, et al. (2011) Fabrication of chitosan/poly(caprolactone) nanofibrous scaffold for bone and skin tissue engineering. Int J Biol Macromol 48: 571-576.
- Pabbruwe MB, Kafienah W, Tarlton JF, Mistry S, Fox DJ (2010) Repair of meniscal cartilagewhite zone tears using a stem cell/collagen-scaffold implant. Biomaterials 31: 2583-2591.
- NdreuHalili A, Hasirci N, Hasirci V (2014) A multilayer tissue engineered meniscus substitute. J Mater Sci: Mater Med 25: 1195-1209.
- Papalia R, Franceschi F, Balzani LD, D’Adamio S, Maffulli N, et al. (2013) Scaffolds for partial meniscal replacement: An updated systematic review. Br Med Bull 107: 19-40.
- Smith BD, Grande DA (2015) The current state of scaffolds for musculoskeletal regenerative applications. Nat Rev Rheumatol 11: 213-222.
- Vrancken ACT, Buma P, Van Tienen TG (2013) Synthetic meniscus replacement: A review. Int Orthop 37: 291-299.
- Elsner JJ, Shemesh M, Shefy-Peleg A, Gabet Y, Zylberberg E, et al. (2015) Quantification of in vitro wear of a synthetic meniscus implant using gravimetric and micro-CT measurements. J Mech Behav Biomed Mater 49: 310-320.
- Cengiz IF, Pereira H, Espregueira-Mendes J, Oliveira JM, Reis RL (2017) Treatments of Meniscus Lesions of the Knee: Current Concepts and Future Perspectives. Regen Eng Transl Med 3: 32-50.
- Myers KR, Sgaglione NA, Goodwillie AD (2014) Meniscal scaffolds. J Knee Surg. 27: 435-442.
- Moran CJ, Busilacchi A, Lee CA, Athanasiou KA, Verdonk PC (2015) Biological augmentation and tissue engineering approaches in meniscus surgery. Arthroscopy 31: 944-955.
- Warth RJ, Rodkey WG (2015) Resorbable collagen scaffolds for the treatment of meniscus defects: a systematic review. Arthroscopy 31: 927-941.
- Scotti C, Hirschmann MT, Antinolfi P, Martin I, Peretti GM (2013) Meniscus repair and regeneration: review on current methods and research potential. Eur. Cell Mater 26: 150-170.
- Tucker B, Khan W, Al-Rashid M, Al-Khateeb H (2012) Tissue engineering for the meniscus: a review of the literature. Open Orthop J 6: 348-351.
- Kremer A, Ribitsch I, Reboredo J, Dürr J, Egerbacher M, et al. (2017) 3D co-culture of meniscal cells and mesenchymal stem cells in collagen type I hydrogel on a small intestinal matrix a pilot study towards equine meniscus tissue engineering. Tissue Eng Part A 23: 390-402.
- Ndreu Halili A, Hasirci N, Hasirci V (2013) A mechanically functional collagen-based construct designed as e meniscus substitute. J Biomat Tissue Eng 3: 173-184.
- Zhang H, Leng P, Zhang J (2009) Enhanced meniscal repair by overexpression of hIGF-1 in a full thickness model. Clin Orthop Relat Res 467: 3165-3174.