Research Article, J Aging Geriatr Med Vol: 1 Issue: 2
Cuprizone-Induced Disorders of Central Nervous System Neurons, Behavioral Reactions, Brain Activity of Macrophages and Antioxidant Enzymes in the Mice of Different Ages: Role Leukemia Inhibitory Factor in Their Improvement
Labunets IF1*, Melnyk NO1,2, Rodnichenko AE1, Rymar SE1 and Utko NA1
1Cell and Tissue Technologies Department, State Institute of Genetic and Regenerative Medicine, NAMS of Ukraine, Keiv, Ukraine
2Histology and Embriology Department, OO Bogomoletz National Medical University, Kiev, Ukraine
*Corresponding Author : Irina Labunets
Cell and Tissue Technology Department, State Institute of Genetic and Regenerative Medicine, National Academy of Medical Sciences of Ukraine, Ukraine
Tel: +38044 4687550
Fax: +38044 4687541
E-mail: irina_labunets@ukr.net
Received: February 10, 2017Accepted: February 12, 2017 Published: February 19, 2017
Citation: Labunets IF, Melnyk NO, Rodnichenko AE, Rymar SE, Utko NA (2017) Cuprizone-Induced Disorders of Central Nervous System Neurons, Behavioral Reactions, Brain Activity of Macrophages and Antioxidant Enzymes in the Mice of Different Ages: Role of Leukemia Inhibitory Factor in their Improvement. J Aging Geriatr Med 1:2.
Abstract
Objective: The present work aimed at studying neuroprotective effects of recombinant human leukemia inhibitory factor (rhLIF) on mice of different ages with cuprizone model of demyelination.
Methods:In the 129/Sv mice at 3-5 and 16-17 months of age we assessed (a) motor and emotional activity in the “open field” test and (b) activity of brain antioxidant enzymes and macrophages capable to phagocytosis of latex beads. After staining histological sections of the brain and spinal cord toluidin blue we determined the percentage of neurons with unmodified, moderate and severe structural changes. Cuprizone was fed daily for 3 weeks. RhLIF was injected after 7-days cuprizone diet, one administration daily, 50 µg/kg.
Results: In the cuprizone-treated mice of both age groups, the percentage of neurons with severe changes in the brain and spinal cord were increased. In the young and aged animals receiving cuprizone and rhLIF the amounts of neurones with destructive changes were reduced, being less pronounced in aged mice. Cuprizone decreased the amounts of crossed squares and faecal boluses in the mice of both age groups. RhLIF restored emotional activity in these mice, but the increase of motor activity was observed only in young mice. In the brain of cuprizone-treated mice of both age groups the activity of catalase and glutathione peroxidase (GP) inhibits; the changes were more pronounced in the aged mice. Positive effects of rhLIF on GP activity were seen only in the young mice. The percentage of active macrophages was increased in cuprizone-treated mice of both age groups. Decrease of amount and activity of macrophages after injections of the rhLIF was observed only in the aged animals.
Conclusion: LIF may be a perspective neuroprotective drug in multiple sclerosis. The efficiency of LIF in multiple sclerosis can be raised if the data about its role in aging are taken into account.
Keywords: Age; cuprizone; LIF; Neuron; Behavioral reactions; Macrophages; Antioxidant enzymes
Introduction
Multiple sclerosis is one of the most spreading demyelinating disease of the central nervous system (CNS) [1,2]. This disease is characterized by the destruction of the myelin in nerve fibers and, as a result, disturbance of nerve impulse transmission and motor activity suppression. However, more and more researchers consider multiple sclerosis as a neurodegenerative disease [3]. Damage of neurons may contribute to the changes of their functioning and the formation of such neurological symptoms of multiple sclerosis as disturbances of memory, emotions, intellect, vegetative disorders, etc.
Although multiple sclerosis generally occurs at younger years, today it can be registered after the age of 45 years. In older age this disease has mostly infectious and toxic nature, progressive character and more severe clinical symptoms [4]. The investigation of agerelated peculiarities of multiple sclerosis pathogenesis requires the use of its adequate experimental models.
Cuprizone is a copper-chelating agent that in animals (mouse, rat) induces toxic effect on mature myelin-producing oligodendrocytes [5-7]. So, the decrease of cytochrome oxidase and monoamine oxidase activities in the mitochondria of oligodendrocytes is accompanied by their apoptosis and, as a result, demyelination in CNS of cuprizonetreated animals. In addition, the neuroinflammation and oxidative stress in the CNS are the important mechanisms of nerve cell damage in cuprizone model of multiple sclerosis [8]. Several authors have registered with increases of the amount of apoptotic oligodendrocytes, the content of malondialdehyde and the activation of macrophages and microglia cells producing proinflammatory cytokines (tumor necrosis factor-α and interferon-γ) in the brain of cuprizone-treated young mice [9,10]. However, the importance of the changes in activity of factors of neuroinflammation and antioxidant defense system for the development of the structural changes in CNS neurons both in young and aged animals with experimental model of multiple sclerosis, remains insufficiently studied.
Several authors have revealed definite age-related specifics of the influence of cell factors (cytokines, growth factors) on the CNS regeneration after damage as aging is associated with changes in neurogenesis and sensitivity of the nerve cells caused by these factors [11-13]. There are published data indicating that leukemia inhibitory factor (LIF) is both polyfunctional cytokine and neurotrophical factor [14,15]. In other words, this cytokine is the growth factor for neural stem cells (NSCs) [16]. LIF increases proliferation of the precursors of oligodendrocytes, their differentiation into mature oligodendrocytes and expression of basic protein myelin in the brain cortex of young cuprizone-treated mice [17,18]. This cytokine has an antiapoptotic effect on the oligodendrocytes, NSCs and neurons. It increases the regenerative potential of the motoneurons after damage [12,19]. The expression of LIF in the CNS is increased in the young cuprizone-treated mice, but older mice with cuprizone model are characterized by delayed gene expression of brain growth factors [12,20]. Age-related disturbances of the functioning of the oligodendrocytes, NSCs and neurons may contribute to the decrease of both, regenerative potential of nervous tissue after damage and efficiency of neuroprotective drugs [11,20,21].
Therefore, our experiments were undertaken to determine protective effects of LIF on the structure of the CNS neurons with involvement of brain macrophages and antitioxidant enzymes in the mice of different age with an experimental cuprizone model of multiple sclerosis. As a result of experimental investigations, new data will hopefully be received on: (a) the mechanisms of neuroprotective effect of LIF realized via changes of pathogenic factors of multiple sclerosis development; and (b) age as modifying factor of cytokine influence on post-damage CNS regeneration.
Material and Methods
Animals
The research was conducted on female 129/Sv mice (genotype H-2b) aged 3-5 months (n=42) and 16-17 months (n=42). According to our investigations, 129/Sv mice are sensitive to the toxic action of neurotoxin cuprizone [22]. All animals were kept under a light/dark schedule (12:12) with food and water ad libitum at the experimental clinic of State Institute of Genetic and Regenerative Medicine NAMS of Ukraine. Mice were housed in groups of three or four animals under controlled parameters (temperature and humidity). Biological tissue were reseived at 9:00 a.m. by ether anesthesia of mice. All experiments with animals were carried out according to the Law of Ukraine “About protection of animals from cruelty” (from 21.02.2006), the European Convention for the Protection of Vertebrate Animals used for experimental and other scientific purposes (European Convention, Strasburg, 1986).
Cuprizone-induced demyelination
Mice of 3-5 months and 16-17 months of age received the neurotoxin cuprizone [bis(cyclohexylidenehydrazide)] ("Sigma", USA). For cuprizone-induced demyelination, mice were fed ad libitum a food mixed with cuprizone at 0.2% (w/w), daily, for 3 weeks. Normal (intact) mice were used normal food. The body weigth of each mouse was measured before and after using neurotoxin or cuprizone-free food. The loss of weight is one of the manifestations of the toxic effects of cuprizone. Mice of both age groups on the cuprizone diet lost weight (p<0.05); weight of normal mice was without changes (data not shown).
Recombinant human LIF (rhLIF)
In the research we used the rhLIF. Its biological effect on both human and murine cells is known; beside, rhLIF after administration to mice interacts with cytokine receptors on neural cells [23]. For cloning of rhLIF gene from human genome the method of recombinant deoxyribonucleic acid (DNA) was applied [15]. Ribonucleic acid derived from the human placenta was further used as a template in reverse transcription and then amplified with polymerase chain reaction. Obtained LIF complementary DNA was cloned in pUC18 and sequenced. After its subcloning in pET24 expressing vector the biological activity of the produced protein was tested on M1 leukemia myeloid line. LIF has been identified as the differentiation factor of this cell line. Treatment with this protein of M1 cells leads to the differentiation of malignant cells, which is the proof that the cloning complementary DNA is the recombinant gene of LIF. Obtained protein by expression of the cloned gene in cells E.coli Bl21 (DE3) RP, was solubilized, refolded and used in the study on its effect on the pathology of the nervous system.
Administration of rhLIF cuprizone-fed mice
Cytokine was injected to mice of different ages intraperitoneal, one administration daily, 50 μg/kg of body weigh, after 7 days of the use cuprizone, (14 injections). This scheme of injections is based on literature data [24] and our results of changes in structure of CNS neurons and motor activity in young mice after 7 days of cuprizone treatment [25]. The control groups of mice different ages with cuprizone model were received a similar scheme injections of phosphate buffered saline (PBS).
Experimental groups
There were next groups: (i) normal (intact) mice of different ages, which were fed normal food for 3 weeks; (ii) mice of different ages, which were received cuprizone-containg food for 3 weeks and injections of PBS; (iii) mice of different ages, which were received a cuprizone-containg food and injections of rhLIF. Fourteen mice were used for each experimental group of aged 3-5 months and 16-17 months.
Morphological studies
For morphological studies of the structures of the CNS (cortex, cerebellum, lumbar spinal cord) in mice used staning histological sections cresyl violet and toluidine blue (by Nìssl) [22]. Cresyl violet to determine the changes in myelin sheets of nerve fibers. Toluidine blue selectively binds to membrane structures, allowing to diagnose structure of nucleus and cytoplasm of neuron. Carried out the standard processing the researched tissue of the CNS: with the obtained paraffin blocks was made histological slices the thickness of 5-7 microns using automatic rotary microtome.
The animals were evaluated for morphometric analysis determined the percentage of neurons with unmodified, and with moderate and severe structural changes (staining of histological specimens of toluidine blue) [22]. Detected structures were observed to change of shape the cell bodies and the nucleus of neurons, locations of Nissl substance. Moderate changes in neurons are reactive to damage and characterized by displacement nucleolus to nuclear membrane, and an increase in the size of the nucleus. Cytoplasm of neurons was hypochrome, Nissl substance is not defined. Severe structural changes of neurons are destructive and characterized by a decrease in the size of the nucleus with change of the shape, the nucleolus is not visible. Size of cell bodies of neurons is considerably reduced and they were hyperchrome .
Behavioral reactions
Behavioral reactions in mice were studied in "open field" test, which is one of the adequate approach for estimation of motor disorders after use of neurotoxins [26]. The "open field" test also allows to estimate of emotional animal activity [27,28]. The mice have brain neurochemical and molecular mechanisms of emotional behavior, similar to those in humans [29].
In our study testing procedure in the "open field" test as follows: individual mice were placed in a chamber with high edges (60 cm × 60 cm × 60 cm). The floor of chamber had similar sectors (16 pieces) that are designed for the visual registration of horizontal motor activity of experimental animals (the amount of crossed squares). Emotional activity of mice was estimated by number of faecal boluses. The number of crossed squares and faecal boluses were counted during 3 min.
Activities of antioxidant enzymes
Activities of antioxidant enzymes were assessed in the supernatants of brain homogenates (cerebellum) (10000 g for 20 min) with μQuant Bio-Tek spectrophotometer (USA) [30-33]. To investigate the activity of superoxide dismutase (SOD) (EC 1.15.1.1) we used a method based on the ability of SOD to inhibit the autooxidation reaction of adrenaline ("Fluka", Germany) to adrenochrome at pH 10.2. SOD activity was expressed in units per 1 mg of protein for 1 min. Catalase (EC 1.11.1.6) activity was determined with the ÐÂÂ2ÐÂÂ2 destruction kinetics (“Riedel-deHaën”, Germany) and expressed in μmol recycled ÐÂÂ2ÐÂÂ2 per 1 mg protein for 1 min. The activity of glutathione peroxidase (GP) (EC 1.11.1.9) was measured by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidation (“Sigma”, USA) in the glutathione reductase reaction and expressed in nmol NADPH oxidized per 1 mg of protein for 1 min. The protein content of the brain homogenates was measured by the Lowry method. Lowry method is based on the reaction of protein residues with copper under alkaline conditions. The resulted compound is reduced by Folin–Ciocalteu reagent producing a blue coloured product measured at 750 nm.
Functional activity of brain macrophages/phagocytic cells
“Brain macrophages” are heterogenous population [34]. Among of them microglial cells and blood-derived macrophages have similar parameters, for example the possibility to phagocytous of latex beads. In mice the activity of brain macrophages/phagocytic cells was determined in accordance with the recommendations of Jordan et al. [35] and Stephanov [36], in our modification. The method is based on the quantitative determination of the latex beads that are phagocytosed by active macrophages after joint incubation. The brain gomogenates received in PBS. The cell suspension passed through the cell filters with a pore diameter of 100 μm, and then taken to the cups of Petri with a diameter of 100 mm in the culture medium such composition: RPMI-1640, 10% fetal bovine serum (FBS), antibiotics (100 IU/ml penicillin, 100 g/ml streptomycin), 2 mM L-glutamine. The cell suspension cultivated for 1 hour at 37°Ð¡ in gas mixture of 5% СÐÂÂ2. Then adherent cells were dissociated with 0.05% trypsin in 0.53 mM Na2 EDTA. 10% FBS was added to the cells for trypsin action inhibition. Then 0.2 ml of this suspension (2.5 × 106 ml) put on microscope slides for cover and are incubated for 1 hour in standard conditions. After incubation at the resulting monolayer add 0.2 ml suspension latex (2.5 × 108 ml) in RPMI-1640 medium and incubating 45 min at 37оС in gas mixture of 5% СÐÂÂ2. (All reagents - "Sigma", USA). Then the cells were fixed and stained with Romanovsky- Gimsa (“Macrochim”, Ukraine). In the light microscope were counted at 200 macrophages/phagocytic cells and defined: phagocytic index (the percentage of cells capable of phagocytosis of latex beads) and the phagocytic activity (the amount of latex beads, which phagocytoused by one macrophage/phagocytic cell).
Statistical Analysis
Statistical analysis of the results was carried out using the Student’s t- test [37]. Differences between the means of comparable groups were significant at p<0.05. For statistical analysis were used the program Statistica 7.0. Copyright © StatSoft.Inc.1984-2004
Results
Influence of exogenous rhLIF on the structural changes in the CNS of mice different ages with cuprizone-induced demyelination
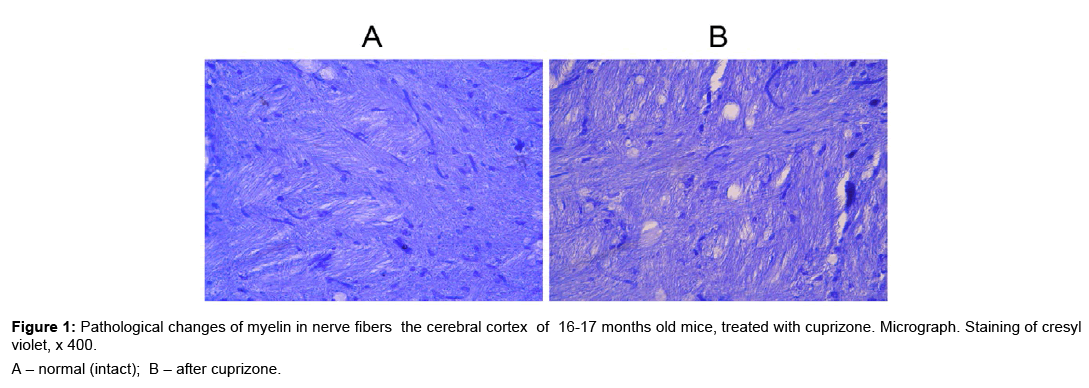
The demyelinating effect of cuprizone on CNS of young mice has been shown [5,6]. During demyelination myelin destruction and welling between mesaxone layers were observed. We recognized the toxic effect of cuprizone on the myelin membrane of axons neurons in the brain of 16-17 months old mice (Figure 1A,B). During the morphological studies of cerebral cortex we have identified areas with nerve fibers in condition of demyelination (Figure 1B). The histological specimens, which were stained of cresyl violet, areas with demyelination of nerve fibers appear as light formations that resemble the scum.
In the 16-17 months old versus intact mice, the cuprisone receiving animals demonstrated pathologically modified neurons in the brain and spinal cord. (Figure 2A,B,D,E,G,H). After morphometric analysis of histological specimens revealed that the structural changes in the neurons of gray substance the brain (cortex, cerebellum) and the lumbar spinal cord of young cuprizone-treated mice have a different characteristics from reactive (moderate) to severe (destructive) (Table 1). Thus, in cuprizone-treated mice, significantly decreases the percentage of neurons unmodified in all the structures of the CNS, and, on the contrary, increases the percentage of neurons with severe changes.
| Age and group of mice | Unmodified | Moderate changes | Severe changes | |
|---|---|---|---|---|
| Neurons of cortex of cerebrum | ||||
| 3-5 months of age | ||||
| Normal (intact) | 88 | 12 | 0 | |
| Cuprizone | 8 | 23 | 69 | |
| Cuprizone + rhLIF | 28 | 64 | 8 | |
| 16-17 months of age | ||||
| Normal (intact) | 82 | 18 | 0 | |
| Cuprizone | 24 | 44 | 32 | |
| Cuprizone + rhLIF | 67 | 24 | 9 | |
| Neurons of ganglion layer of cerebellum | ||||
| 3-5 months of age | ||||
| Normal (intact) | 86 | 14 | 0 | |
| Cuprizone | 9 | 27 | 64 | |
| Cuprizone + rhLIF | 40 | 43 | 17 | |
| 16-17 months of age | ||||
| Normal (intact) | 81 | 19 | 0 | |
| Cuprizone | 21 | 45 | 34 | |
| Cuprizone + rhLIF | 76 | 24 | 0 | |
| Neurons of lumbar spinal cord | ||||
| 3-5 months of age | ||||
| Normal (intact) | 93 | 7 | 0 | |
| Cuprizone | 2 | 4 | 94 | |
| Cuprizone + rhLIF | 13 | 71 | 16 | |
| 16-17 months of age | ||||
| Normal (intact) | 92 | 8 | 0 | |
| Cuprizone | 3 | 5 | 92 | |
| Cuprizone + rhLIF | 8 | 56 | 36 | |
Table 1: The influence of cuprizone and rhLIF on morphological changes of neurons in the brain and spinal cord of mice different ages (%).
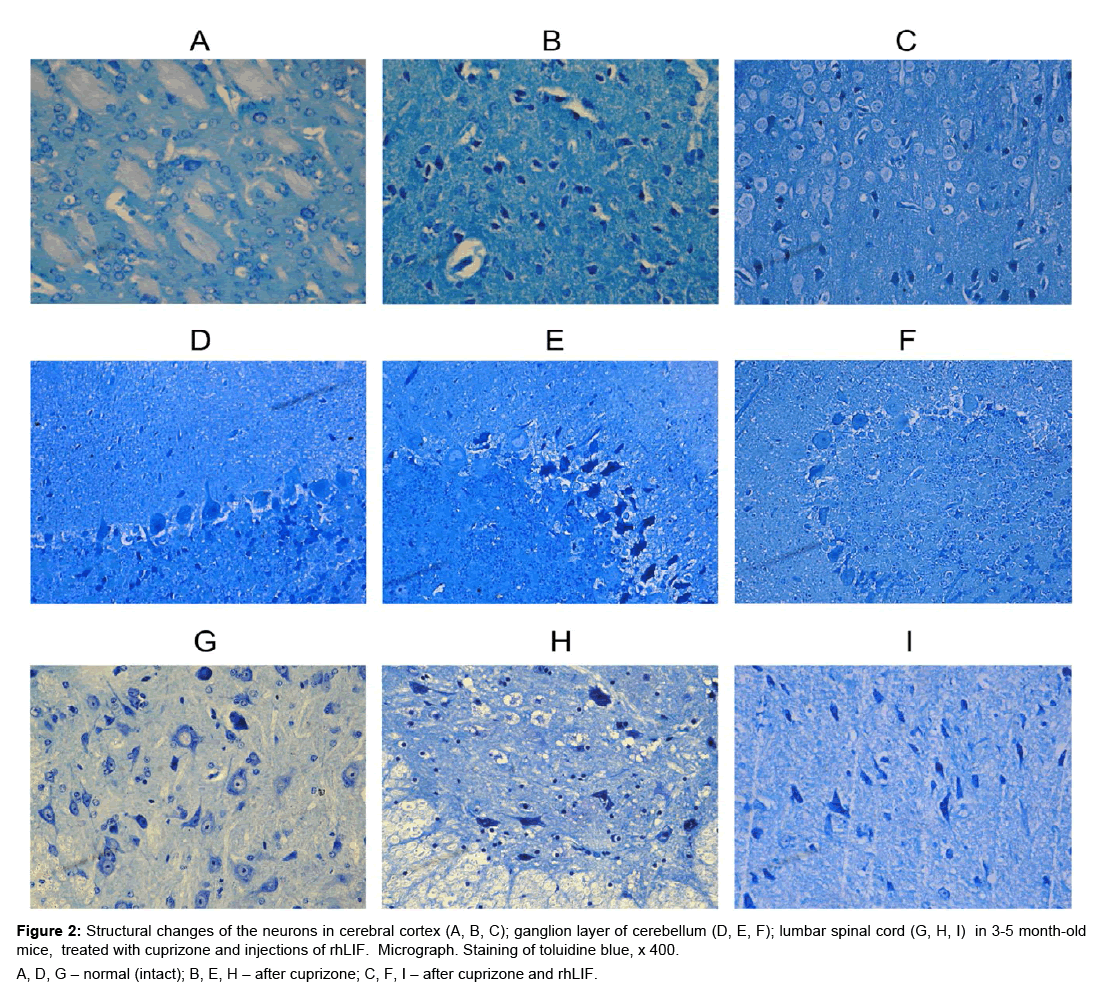
Figure 2: Structural changes of the neurons in cerebral cortex (A, B, C); ganglion layer of cerebellum (D, E, F); lumbar spinal cord (G, H, I) in 3-5 month-old mice, treated with cuprizone and in jections of rhLIF. Micrograph. Staining of toluidine blue, x 400.
A, D, G – normal (intact); B, E, H – after cuprizone; C, F, I – after cuprizone and rhLIF.
We found that in 16-17 months old mice after the use of cuprizone in the brain and spinal cord are pathologically modified neurons compared with normal (intact) mice (Figure 3A,B,D,E,G,H). The number of neurons with destructive changes in aged cuprizonetreated mice, is lower in the cerebral cortex and the cerebellum, versus of the young experimental mice (Table 1). In the spinal cord of cuprizone-treated mice, the number of neurons with reactive and destructive changes does not depend on animals age.
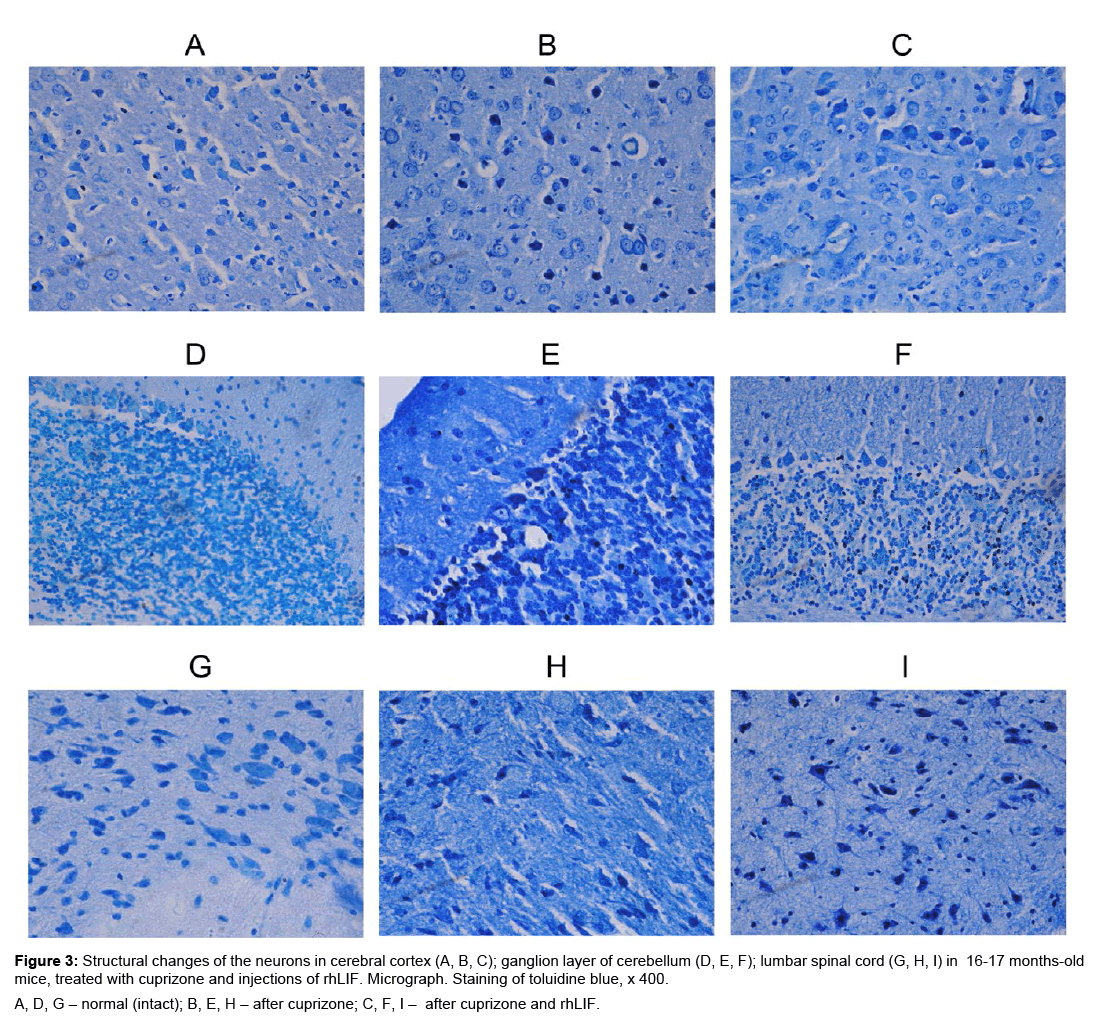
Figure 3: Structural changes of the neurons in cerebral cortex (A, B, C); ganglion layer of cerebellum (D, E, F); lumbar spinal cord (G, H, I) in 16-17 months-old mice, treated with cuprizone and injections of rhLIF. Micrograph. Staining of toluidine blue, x 400.
A, D, G – normal (intact); B, E, H – after cuprizone; C, F, I – after cuprizone and rhLIF.
In young mice, after administration of cuprizone and rhLIF, was observed light cytoplasm in neurons, increased the size of nucleus and nucleolus (Figure 2C,F,I). These changes are indices of the active state of the neurons and indicate the development of regenerative, adaptive reactions after use cuprizone and rhLIF. In young animals after injections of rhLIF increases the percentage of unmodified neurons and reduces the percentage of neurons with destructive changes (Table 1). The positive effect of cytokìne is most pronounced in the cortex of cerebrum (Table 1). In 16-17 months old mice after use of cytokine in the brain and the spinal cord decreases the percentage of neurons with severe structural changes (Table 1).
In general, the obtained data indicate the positive effect of rhLIF on the synthetic processes in the neurons of CNS both in young and aged cuprizone-treated mice. However, the regenerative processes under the influence of rhLIF better in young animals.
Influence of exogenous rhLIF on the behavioural reactions in mice of different ages with cuprizone-induced demyelination
Motor activity: Our study has shown that horizontal motor activity decreased significantly in the mice of both age groups treated with cuprizone (p<0.05 vs norm) (Table 2). After injections of the rhLIF to experimental young mice their motor activity increased (p<0.05) though it remained still low in comparison with normal (intact) animals (p<0.05) (Table 2). The 16-17 months old mice treated with cuprizone and cytokine did not show any changes in their motor activity (p>0.05).
| Age and group of mice | Amount of crossed squares | Amount of fecal boluses |
|---|---|---|
| 3-5 months of age | ||
| Normal (intact) | 60.0 ± 5.6 | 1.9 ± 0.3 |
| Cuprizone | 10.0 ± 1.8 * | 1.0 ± 0.2* |
| Cuprizone + rhLIF | 25.0 ± 2.9 *# | 2.2 ± 0.4# |
| 16-17 months of age | ||
| Normal (intact) | 50.0 ± 4.4 | 2.7 ± 0.3 |
| Cuprizone | 11.0 ± 1.5* | 0.8 ± 0.2* |
| Cuprizone + rhLIF | 13.5 ± 3.7*& | 2.0 ± 0.4# |
Table 2: The indices of behavioral reactions (“open field” test) in mice of different ages after the use of cuprizone and rhLIF, M ± m.
Emotional activity: After use of neurotoxin the amount of faecal boluses was significantly decreased in the mice of both age groups (p<0.05 vs norm) while after rhLIF administration it was increased (p<0.05 vs cuprizone) (Table 2).
In summing up, cuprizone significantly suppressed motor and emotional activities both in the young and 16-17 months old mice. Administration of the rhLIF restored excited emotional activity in experimental mice of both age groups. However increase of motor activity was observed only in the young animals.
Influence of exogenous rhLIF on the activity of antioxidant enzymes in the cerebellum of mice different ages with cuprizone-induced demyelination
In our study we investigated activities of the main enzymes of the antioxidant defense system in reactive oxygen species’ (ROS) neutralization, such as SOD, catalases and GP [38]. Activation of SOD is the first link of antioxidant protection. This enzyme converts superoxide anion-radical into electro-neutral form ÐÂÂ2O2, whose conversion to ÐÂÂ2O is controlled by the catalase and GP. In the cerebellum of young mice with cuprizone diet the SOD activity remained practically unchanged (p>0.05 vs norm). However, in the 16-17 months old mice it was elevated and became higher compared with young experimental mice (p<0.05) (Table 3).
| Age and group of mice | Superoxide Dismutase |
Catalase | Glutathione peroxidase |
|---|---|---|---|
| 3-5 months of age | |||
| Normal (intact) | 12.9 ± 0.3 | 1.4 ± 0.1 | 8.8 ± 0.5 |
| Cuprizone | 12.7 ± 0.1 | 1.3 ± 0.1 | 7.5 ± 0.1* |
| Cuprizone + rhLIF | 13.5 ± 0.3 | 1.4 ± 0.2 | 8.5 ± 0.2# |
| 16-17 months of age | |||
| Normal (intact) | 12.8 ± 3.0 | 2.0 ± 0.2 | 8.1 ± 0.9 |
| Cuprizone | 17.6 ± 2.1& | 1.5 ± 0.1* | 5.3 ± 0.6*& |
| Cuprizone + rhLIF | 16.9 ± 1.5& | 1.5 ± 0.1* | 4.8 ± 0.6*& |
Table 3: Activity of antioxidant enzymes in cerebellum of mice different ages after the use of cuprizone and rhLIF, M ± m.
After the use of cuprizone the cerebellum catalases activity decreased significantly only in the aged mice (p<0.05 vs norm) (Table 3). The activity of GP was suppressed in cuprizone-treated mice of both age groups (p<0.05 vs norm), being more intensive in 16-17 months old mice (p<0.05 vs young) (Table 3). Injections of rhLIF restored the GP activity in the young experimental mice (p<0.05 vs cuprizone) (Table 3).
Thus, cuprizone suppresses antioxidant enzyme activity in the cerebellum of mice of different ages; changes of this activity are more marked in the aged mice. A positive effect of rhLIF on antioxidant enzyme activities is seen only in the young mice.
Influence of exogenous rhLIF on the activity of brain macrophages/phagocytic cells in mice of different ages with cuprizone-induced demyelination
Proportion of active macrophages/phagocytic cells significantly increased in mice of both age groups after the use of cuprizone, but their functional activity is only in the aged mice (p<0.05 vs norm) (Table 4). After the injections of rhLIF we observed the decrease of studied indices only in experimental aged mice (p<0.05 vs cuprizone) (Table 4).
| Age and group of mice | Phagocytic index (%) | Phagocytic activity |
|---|---|---|
| 3-5 months of age | ||
| Normal (intact) | 89.1 ± 1.7 | 3.6 ± 0.2 |
| Cuprizone | 94.6 ± 1.6 * | 3.4 ± 0.7 |
| Cuprizone + rhLIF | 94.3 ± 1.6 * | 3.7 ± 0.3 |
| 16-17 months of age | ||
| Normal (intact) | 84.5 ± 0.9& | 4.3 ± 0.6 |
| Cuprizone | 97.5 ± 0.9* | 5.6 ± 0.1*& |
| Cuprizone + rhLIF | 91.6 ± 2.1*# | 3.9 ± 0.1# |
Table 4: Activity of macrophages/phagocytic cells in brain of mice different ages after the use of cuprizone and rhLIF, M ± m.
Thus, under the influence of cuprizone in mice of both age groups observed the increase of amount and activity brain macrophages/ phagocytic cells: more intensive changes were in older mice. Inhibition the activity of macrophages/phagocytic cells after rhLIF injections was registered only in 16-17 months old mice.
Discussion
According to our investigation, ÑÂÂuprizone increases the amount of neurons with destructive changes in the cortex of cerebrum, cerebellum and in spinal cord of young mice. Destructive changes in the neurons of CNS in cuprizone-treated mice may be the result of oxidative stress and decreased activity of the antioxidant enzymes. Apoptosis of the oligodendrocytes, increase of the malondialdegide and hydrogen peroxide levels and, opposite, decrease of the SOD, catalase and GP activities in the brain have been demonstrated in the young cuprizone-treated mice [7,10]. We observed a correlation between the decrease of brain GP activity and destructive changes of the brain neurons in the young mice.
Activation of microglial cells/macrophages in the CNS of young mice after the use of cuprizone is another possible way for the development of degenerative changes in the nerve cells. According to Liu et al. [39], microglial cells of the brain in cuprizone-treated mice produce a lot of ROS promoting damage of oligodendrocytes and myelin. In our study, following 3-weeks use of cuprizone we observed an increase in the number of active macrophages/phagocytic cells in the brain of young mice. However, increase of their activity took place much earlier – 7 days after neurotoxin use (unpublished data). Alongside, at this period we registered moderate structural changes in the neurons of cerebral cortex and cerebellum, as well as a significant decrease of the horizontal motor activity [25].
Thus, in our young mice with cuprizone-induced demyelination we attributed changes in the activity of macrophages/phagocytic cells and antioxidant enzymes to the development of damages not only of the oligodendrocytes but also of the neurons leading to the decrease of motor and emotional activities.
After injections of the rhLIF to young cuprizone-treated mice we observed the decrease of the share of damaged neurons in the CNS, the increase of the share of unmodified neurons as well as motor and emotional activities. Positive changes in the study indices may be linked to the stimulating influence of cytokine on the formation of oligodendrocytes and myelin synthesis, proliferation of brain NSCs and their differentiation in neurons, antiapoptotic effect [12,16,17,40]. According to our results, the activity of antioxidant enzymes increases after rhLIF injections in the brain of young cuprizone-treated mice.
In the 16-17 months old mice we observed some differences in the structural changes of the brain neurons both in the intact and cuprizone and rhLIF-treated mice. Thus in normal (intact) aged mice, the number of unmodified neurons decreases and the number of neurons with moderate changes increases both, in the cerebral cortex and cerebellum. In the 13-14 months old mice, correlation of changes of the neurogenesis and increased proliferation and activity of the brain microglia cells has been shown [41,42]. We also observed higher activity of brain macrophages/phagocytic cells in the normal (intact) aged versus young mice.
Our data obtained in the 16-17 months old mice with cuprizoneinduced demyelination have shown less marked changes of the brain neuronal structure in comparison with young animals. In the opinion of other investigators, the nerve cells undergo age changes of sensitivity under effects of other damaging factors, e.g. cuprizone [6,11]. Besides, the brain neurons of older cuprizone-treated mice can form delayed reaction to damage factors. According to our investigations, the aged mice did not display any activation of brain macrophages/phagocytic cells after 7 days of cuprizone treatment (unpublished data), rather 3 weeks later (Table 4).
In the nerve cells of older mice the expression of LIF decreases and differentiation of the oligodendocytes delays [20]. In our study of 16-17 months old mice receiving cuprizone and rhLIF, we observed the decrease of the percentage of neurons with moderate and severe structural changes. Positive brain structural changes were paralleled by the decreased brain activity and reduced number of macrophages/ phagocytic cells and elevated behavioral reactions. However, positive effects of rhLIF on structural changes of brain neurons were lesser in the comparison with young mice. Consequently, this can be linked to age-related changes of neurogenesis as well as sensitivity of nerve cells to the regulatory factors.
Conclusion
The followings have been found:
Development of structural changes in neurons of CNS in cuprizone-treated young and aged mice. Neurodegenerative changes in 16-17 months old mice indicate the importance of using not only of the young but also of the older mice in the investigation of multiple sclerosis pathogenesis.
The possible ways of development of changes in the structure of CNS neurons in cuprizone-treated mice of different ages with an involvement of brain macrophages/phagocytic cells and antioxidant defense factors.
Neuroprotective effects of rhLIF in cuprizone-treated mice of different ages are realized by the way of changing activities of brain macrophages/phagocytic cells and antioxidant enzymes. Effects of exogenous rhLIF depend on animal age. The efficiency of LIF in multiple sclerosis can be raised if the data about its role in aging are taken into account.
Our findings may contribute to the knowledge about the influence of cytokines (LIF) on regenerative potential of the CNS after damage in aging that benefit their further application in regenerative medicine.
References
- Loma I, Heyman R (2011) Multiple sclerosis: pathogenesis and treatment. Curr Neuropharmacol 9: 409-416.
- Mishchenko TS, Shulga OD, Bobryk NV, Shulga LA (2014) Multiple sclerosis: global perspectives. Ukr Med Chasopys 101: 84-87.
- Wegner C, Esiri MM, Chance SA, Palace J, Matthews PM (2006) Neurocortical neuronal, synaptic and glial loss in multiple sclerosis. Neurology 67: 960-967.
- Ismailov MG, Shevchenko PP, Yaschenko IA (2014) Multiple sclerosis and debut in the elderly. Adv Curr Nat Sci N6: 122-123.
- Acs P, Kalman B (2012) Pathogenesis of multiple sclerosis: what can we learn from the cuprizone model. Methods Mol Biol 900: 403-431.
- Kipp M, Clarner T, Dang J, Copray S, Beyer C (2009) The cuprizone animal model: new insights into an old story. Acta Neuropathol 118: 723-736.
- Acs PI, Selak MA, Komoly S, Kalman B (2013) Distribution of oligodendrocyte loss and mithohondrial toxicity in the cuprizone-induced experimental demyelination model. J Neuroimmunol 262: 128-131.
- Praet J, Guglielmetti C, Berneman Z, Van der Linden A, Ponsaerts P (2014) Cellular and molecular neuropathology of the cuprizone mouse model: clinical relevance for multiple sclerosis. Neurosci Biobehav Rev 47: 485-505.
- Liu J, Tian D, Murugan M, Eyo UB, Dreyfris CF, et al. (2015) Microglia Hv1 proton channel promotes cuprizone-induced demyelination through oxidative damage. J Neurochem 135: 347-356.
- Xuan Y, Yan G, Wu R, Huang Q, Li X, et al. (2015) The cuprizone-induced changes in (1)H-MRS metabolites and oxidative parameters in C57Bl/6 mouse brain: effects of gultiapine. Neurochem Int 90: 185-192.
- Ahlenius H, Visan V, Kokaia M, Lindvall Q, Kokaia Z (2009) Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J Neurosci 14: 4408-4419.
- Gudi V, Gingele S, Skripuletz Th, Stangel M (2014) Glial response during cuprizon-induced de- and remyelination in the CNS: lessons learned. Front Cell Neurosci 8: 73.
- Lindvall O, Kokaia Z (2010) Stem cells in human neurodegenerative disorders – time for clinical translation?. J Clin Invest 120: 29-40.
- Nicola NA, Babon JJ (2015) Leukemia inhibitory factor. Cytokine Growth Factor Rev 26: 533-544.
- Rymar SE, Novikova SN, Irodov DM, Bazalii AV, Sukhorada EM, et al. (2010) Leukemia inhibitory factor: perspectives for use in medicine and development of its producer. J NAMS Ukraine 16: 199-212.
- Buono KD, Vadlamuri D, Gan Q, Levison SW (2012) Leukemia inhibitory factor is essential for subventricular zone neural stem cell and progenitor homeostasis as revealed by a novel flow cytometric analysis. Dev Neurosci 34: 449-462.
- Deverman B, Patterson P (2012) Exogenous leukemia inhibitory factor stimulates oligodendrocyte progenitor cell proliferation and enhances hippocampal remyelination. J Neurosci 32: 2100-2109.
- Schmitz T, Chew LJ (2008) Cytokines and myelination in the central nervous system. Scientific World Journal 2: 1119-1147.
- Li Y, Zang D (2014) The neuron regrowth is associated with the proliferation of neural precurcor cells after leukemia inhibitory factor administration following spinal cord injury in mice. PLoS One 9: e116031.
- Doucette JR, Jiao R, Nazarali AJ (2010) Age-related and cuprizone-induced changes in myelin and transcription factor gene expression and in oligodendrocyte cell densities in the rostral corpus callosum of mice. Cell Mol Neurobiol 30: 607-629.
- Rybachuk O, Pivneva T (2014) Contribution of neural stem cells to regeneration of the central nervous system. Int J Physiol Pathophysiol 5: 83-96.
- Labunets IF, Melnyk NO, Kuzminova IA, Butenko GM (2014) The patent N 94458 UA (G09B 23/28 (2006.01)), “Modeling of the structural changes of the central nervous system neurons in demyelinating diseases”, Ukraine.
- Layton MJ, Lock P, Metcaff D, Nicola NA (1994) Cross-species receptor binding characteristics of human and mouse leukemia inhibitory factor suggest a complex binding interaction. J Biol Chem 2690: 17048-17055.
- Marriott MP, Emery B, Cate HS, Binder MD, Kemper D, et al. (2008) Leukemia inhibitory factor signaling modulates both central nervous system demyelination and myelin repair. Glia 56: 686-698.
- Labunets IF, Melnyk NO, Rymar SYu (2016) The patent N 104976 UA (G09B 23/28 (2006.01)) "Modeling of regeneration of damaged brain neurons during neurodegenerative diseases”, Ukraine.
- Franco-Pons N, Torente M, Colomina MT, Vilella E (2007) Behavioral deficits in the cuprizone-induced murine model of dymyelination/remyelination. Toxicol Lett 30: 205-213.
- Bures J, Bureshova O, Houston DP (1991) Methods and basic experiments for the study of brain and behavior. High school, Moscow.
- Amikishieva AV (2009) Behavioral phenotyping: modern methods and equipment. Vestnik VOGiS 13: 529–542.
- Fisch GS (2007) Animal models and human neuropsychiatric disorders. Behav Genet 37: 1-10.
- Lowry OH, Rosenbrough NH, Farr AL, Randall JR (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275.
- Aebi H (1984) Catalase in vitro. Meth Enzymol 105: 121-126.
- Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70: 158-169.
- Misra HP, Fridovich I (1972) The role of superoxide anion in the autooxidation of epinephrine and simple assay for superoxide dismutase. J Biol Chem 247: 3170-3175.
- Guillemin GJ, Brew BJ (2004) Microglial, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol 75: 388-339.
- Jordan FL, Wynder HJ, Booth PL, Thomas WE (1990) Method for the identification of brain macrophages/phagocytic cells in vitro. J Neurosci Res 26: 74-82.
- Stephanov OV (2001) Preclinical studies of drugs (guidelines). Avicenna, Kiev.
- Lakin GF (1990) Biometrics. High school, ÐÂÂoscow.
- Muradian KK, Utko NA, Mozzhuknina TG, Litoshenko AY, Pishel IN, et al. (2002) Pair-wise linear and 3D nonlinear relationships between the liver antioxidant enzyme activities and the rate of body oxygen consumption in mice. Free Radic Biol Med 33: 1736-1739.
- Liu J, Tian D, Murugan M, Eyo UB, Dreyfris CF, et al. (2015) Microglia Hv1 proton channel promotes cuprizone-induced demyelination through oxidative damage. J Neurochem 135: 347–356.
- Majumder A, Banerjee S, Harrill JA, Machacek DW, Mohamad O, et al. (2012) Neurotrophic effects of leukemia inhibitory factor on neural cells derived from human embryonic stem cells. Stem Cells 30: 2387-2399.
- Hamilton LK, Joppe SE, Cochard L, Fernandes KJ (2013) Aging and neurogenesis in the adult forebrain: what we havelearned and where we should go from here. Eur J Neurosci 37: 1976-1986.
- Moraga A, Pradillo JM, Garcia-Culebras A, Palma-Tortosa S, Ballesteros I, et al. (2015) Aging increases microglial proliferation, delays cell migration, and decreases cortical neurogenesis after f ocal ischemia. J Neuroinflammation 12: 87.