Research Article, J Biochem Eng Bioprocess Technol Vol: 1 Issue: 2
Effect of Speed of Incubator Shaker on Microbial Production of Xylitol
Sri Vani K* and Pydisetty Y
Department of Chemical Engineering, National Institute of Technology, Warangal, Telangana, India
*Corresponding Author : Sri Vani K
Department of Chemical Engineering, National Institute of Technology, Warangal, Telangana, India
Tel: 9848510324
E-mail: vani@nitw.ac.in
Received: May 24, 2018 Accepted: June 22, 2018 Published: June 28, 2018
Citation: Sri Vani K, Pydisetty Y (2018) Effect of Speed of Incubator Shaker on Microbial Production of Xylitol. J Biochem Eng Bioprocess Technol 1:2.
Abstract
The work is an experimental study on the effect of speed of incubator shaker on the microbial production of xylitol from xylose using Candida parapsilosis NCIM-3323 and the optimum values are obtained. The speed of the incubator shaker was varied from 50 rpm to 175 rpm at a temperature of 30oC, initial pH of 3.5 and initial xylose concentration of 60 g/l. It was observed that the maximum production of xylitol was obtained for the speed of incubator shaker of 100 rpm. It was found that maximum xylitol concentration of 14.2 g/l, maximum xylitol yield of 0.284 g/g, maximum xylitol volumetric productivity of 0.5458 g/l-h, maximum xylitol specific productivity of 0.0109 g/g-h were obtained for the speed of incubator shaker of 100 rpm.
Keywords: Batch fermentation; Candida prapsilosis; Yield; Volumetric productivity; Specific productivity; Xylose; Xylitol
Introduction
Xylitol is a five carbon sugar alcohol (1,2,3,4,5 pentahydroxy pentane) with a molecular formula, C5H12O5. The sweetening property matching that of sucrose makes xylitol as a good sugar substitute for food processing industry. It produces perceived sensation of coolness in mouth when it comes in contact with saliva because of its negative heat of solution, which makes it desirable in food products like beverages. As compared to sucrose, xylitol has almost the same sweetness level but has lower caloric content (2.4 Cal/g) than sucrose (4 Cal/g) and is an attractive alternative for diabetics as its metabolism is insulin independent [1]. Xylitol also prevents otitis, osteoporosis, inflammation and is known for its anticariogenic properties in preventing caries and improving oral health [2-4].
Among yeasts, though Candida spp [5-7] has been reported as overproducer of xylitol, these are also suspected to be pathogenic in nature under opportunistic conditions [8,9].
The aim of this study is to develop a biotechnological production process for xylitol and to study the xylitol production characteristics of Candida parapsilosis NCIM-3323. Experiments are carried out by varying speed of incubator as 50, 75, 100, 125, 150 and 175 rpm for production of Xylitol.
Materials and Methods
Microorganism
For the present study the yeast Candida parapsilosis NCIM- 3323 on agar slants was procured from National Chemical Laboratory, Pune. The culture was preserved in a refrigerator at 4°C by periodic subcultures on agar slants.
Preparation of subcultures
Subcultures of yeast are prepared once in a month using agar slants. The chemicals required per 100 ml of distilled water for slants preparation are given in Table 1. All the chemicals are mixed and sterilized in an autoclave for 15 min at a temperature of 120°C and a pressure of 15 psi.
| Component | Malt extract | Glucose | Yeast extract | Peptone | Agar |
|---|---|---|---|---|---|
| Composition g/100 ml | 0.3 | 1.0 | 0.3 | 0.5 | 4 |
Table 1: Chemicals required for slants preparation.
The medium for slant preparation was poured upto 1/3rd of the sterilized test tube kept at an angle of 30° to provide more surface area of the nutrients for the growth of microorganism. After the slants have reached the room temperature, they are exposed to UV light for 30 min to destruct unwanted microorganisms. Then the slants are inoculated with the yeast strain. The slants are incubated at 30°C for a period of two days. After the colonies developed the slants are stored at 4°C.
Seed culture and fermentation medium
Seed culture medium for fermentation was prepared from stock cultures. Composition is given in Table 2. Seed culture was grown in 100 ml of the medium in a 250 ml conical flask at 100 rpm in an incubator shaker for 24 h at 30°C. To prevent the reaction with xylose, (NH4)2SO4 was sterilized separately in an autoclave for 15 min at 15 psi pressure and 120°C temperature to kill the undesirable microorganisms. pH of the medium was adjusted to 6 with acetic acid and KOH. Then the medium was UV sterilized for 30 min. A loopful of organisms is used to inoculate the medium.
| Component | D-xylose | Yeast extract | MgSO4.7H2O | KH2PO4 | (NH4)2SO4 |
|---|---|---|---|---|---|
| Composition g/l | 10 | 2 | 0.4 | 5 | 2 |
Table 2: Chemicals required for seed culture preparation.
Batch fermentation was carried out in 500 ml conical flasks with 350 ml of the fermentation medium with composition given in Table 3, in an incubator shaker at different speeds of the incubator shaker i.e. 50 rpm, 75 rpm, 100 rpm, 125 rpm, 150 rpm, 175 rpm for 120 h by transferring 10 ml of seed culture into the fermentation medium. The fermentation medium was sterilized before inoculation.
| Component | D-xylose | Yeast extract | MgSO4.7H2O | KH2PO4 | (NH4)2SO4 |
|---|---|---|---|---|---|
| Composition g/l | 60 | 2 | 0.4 | 5 | 2 |
Table 3: Chemicals required for fermentation medium.
Analysis
Xylitol was analysed according to the method given in European Pharmacopeia supplement 2000 (page no 1237). Dry cell weight was estimated using 0.22 micron filter paper by taking the difference between initial and final weights of filter paper. Reducing sugar (xylose) content of the medium was estimated using spectrophotometer at 540 nm using dinitrosalicylic acid reagent [10].
Results and Discussion
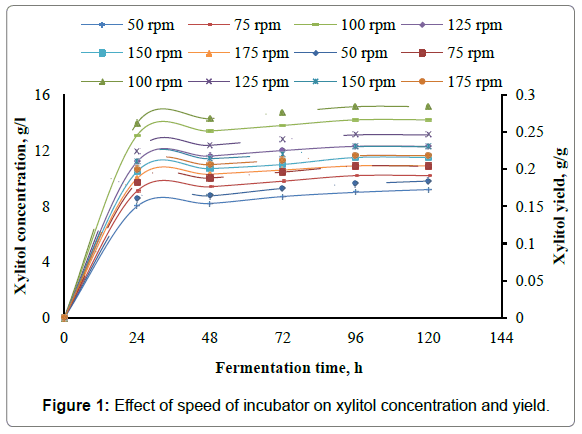
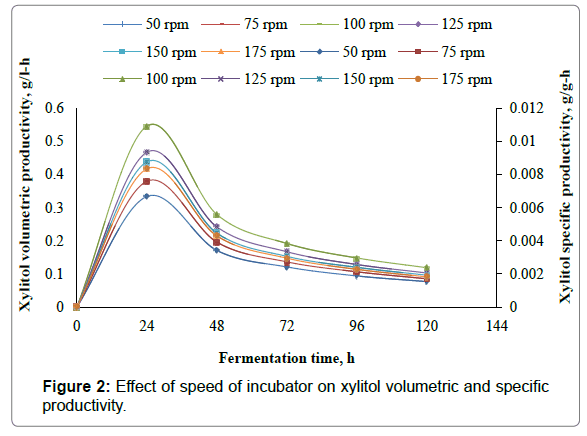
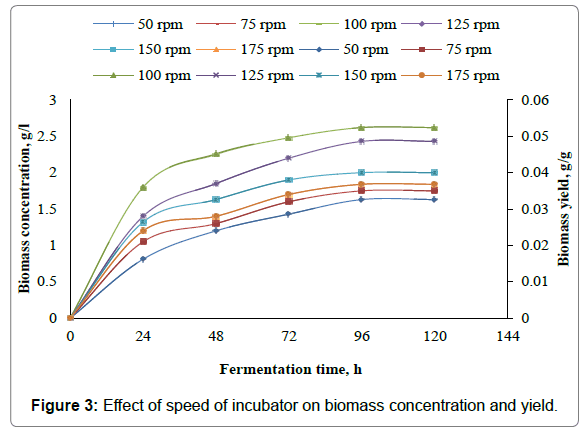
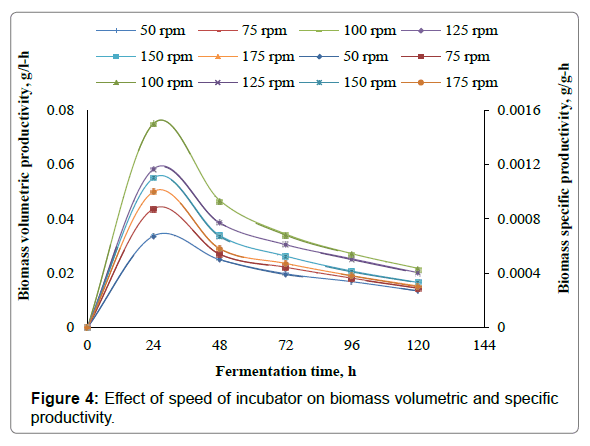
The present work involves the effect of speed of incubator shaker on xylitol production from xylose using Candida parapsilosis NCIM- 3323. To assess the effect of speed of incubator shaker on xylitol production, experiments are carried out in 500 ml Erlenmeyer flasks with 350 ml of the fermentation medium with medium composition given in Table 3. In the present study the speed of the incubator was varied as 50 rpm, 75 rpm, 100 rpm, 125 rpm, 150 rpm and 175 rpm. Initial pH of the samples was adjusted to 3.5, fermentation temperature was set at 30°C. Samples are inoculated with 10 ml, of seed culture of 24 h grown cells of Candida parapsilosis NCIM-3323 in the liquid medium as given in Table 2. Samples are fermented for 120 h in an incubator shaker at different speeds of the incubator shaker. 10 ml of the broth was withdrawn for every 24 h and analyzed for xylitol and biomass dry weight. From the results it was found that maximum xylitol concentration of 14.2 g/l, maximum xylitol yield of 0.284 g/g, maximum xylitol volumetric productivity of 0.5458 g/l-h, maximum xylitol specific productivity of 0.0109 g/g-h were obtained for speed of incubator shaker of 100 rpm. The maximum biomass concentration of 2.62 g/l, maximum biomass yield of 0.0524 g/g, maximum biomass volumetric productivity of 0.075 g/l-h, maximum biomass specific productivity of 0.0015 g/g-h were obtained for speed of incubator shaker of 100 rpm. Figures 1 and 2 represent the effect of speed of incubator shaker on the concentration, yield, volumetric productivity, specific productivity of xylitol, while the Figures 3 and 4 represent the effect of speed of incubator shaker on the concentration, yield, volumetric productivity, specific productivity of biomass. In the figures solid lines represent concentration and volumetric productivity and broken lines represent yield and specific productivity. It was found from present work the maximum productivity and yield of xylitol and biomass were obtained for a speed of incubator shaker of 100 rpm.
Conclusion
The present study involves experimental investigation on the effect the speed of incubator shaker on xylitol production by batch fermentation using Candida prapsilosis NCIM-3323 and it was found that 100 rpm is the best suitable speed of the incubator shaker for xylitol production. From the results it was found that maximum xylitol concentration of 14.2 g/l, maximum xylitol yield of 0.284 g/g, maximum xylitol volumetric productivity of 0.5458 g/l-h, maximum xylitol specific productivity of 0.0109 g/g-h were obtained for speed of incubator shaker of 100 rpm. The maximum biomass concentration of 2.62 g/l, maximum biomass yield of 0.0524 g/g, maximum biomass volumetric productivity of 0.075 g/l-h, maximum biomass specific productivity of 0.0015 g/g-h were obtained for speed of incubator shaker of 100 rpm.
References
- Natah SS, Hussien KR, Tuominen JA, Koivisto VA (1997) Metabolic response to lactitol and xylitol in healthy men. Am J Clin Nutr 65: 947-950.
- Kontiokari T, Uhari M, Koskela M (1995) Effect of xylitol on growth of nasopharyngeal bacteria in vitro. Antimicrob Agents Chemother 39: 1820-1823.
- Mattila P, Knuuttila M, Kovanen V, Svanberg M (1999) Improved bone biomechanical properties in rats after oral xylitol administration. Calcif Tissue Int 64: 340-344.
- Vargas SL, Patrick CC, Ayers GD, Hughes WT (1993) Modulating effect of dietary carbohydrate supplementation on Candida albicans colonization and invasion in a neutropenic mouse model. Infect Immun 61: 619-626.
- Oh DK, Kim SY, Kim JH (1998) Increase of xylitol production rate by controlling redox potential in Candida parapsilosis. Biotechnol Bioeng 58: 440-444.
- Oh DK, Kim SY (1998) Increase of xylitol yield by feeding xylose and glucose in Candida tropicalis. Appl Microbiol Biotechnol 50: 419-425.
- Vandeska E, Amartey S, Kuzmanova S, Jeffries TW (1996) Fed-batch culture for xylitol production by Candida boidinii. Process Biochem 31: 265-270.
- Blandin G, Ozier-Kalogeropoulos O, Wincker P, Artiguenave F, Dujon B (2000) Genomic exploration of the Hemiascomycetous Yeasts: 16. Candida tropicalis. FEBS Lett 487: 91-94.
- Jacques N, Casaregola S (2008) Safety assessment of dairy microorganisms: The hemiascomycetous yeasts. Int J Food Microbiol 126: 321-326.
- Miller GJ (1959) Use of dinitro salicylic acid reagent for determination of reducing sugar. Anal Chem 31: 426-428.