Research Article, J Biodivers Manage Forestry Vol: 8 Issue: 1
How Does Forwarding of Pinus pinea Plantations Affect the Recovery of Plant Assemblages of Stabilised Dunes?
Juan García-de-Lomas1*, Laura Fernández-Carrillo1, María D Cobo2, Ildefonso Martín1, Concepción Saavedra1, Santiago Pérez2, Tomás Ponce2 and Carmen Rodríguez3
1Environment and Water Agency of Andalusia, Seville, Spain
2Conservation Department, Doñana Natural Area, Centro Administrativo El Acebuche s/n, 21760 Matalascañas, Huelva, Spain
3Department of Flora and Fungi, Regional Ministry of Agriculture, Livestock, Fisheries and Sustainable development, Avda Manuel Siurot s/n, 41071, Seville, Spain
*Corresponding Author : Juan García-de-Lomas
Environment and Water Agency of Andalusia, Seville, Spain
Tel: +34.678.623.308
E-mail: juan.garcialomas@juntadeandalucia.es
Received: November 11, 2018 Accepted: February 12, 2019 Published: February 18, 2019
Citation: García-de-Lomas J, Fernández-Carrillo L, Cobo MD, Martín I, Saavedra C, et al. (2019) How Does Forwarding of Pinus pinea Plantations Affect the Recovery of Plant Assemblages of Stabilised Dunes?. J Biodivers Manage Forestry 8:1. doi: 10.37532/jbmf.2019.8(1).208
Abstract
Management of forest plantations towards biodiversity conservation poses a challenge of increasing importance. Felling and subsequent logging have been traditionally used in forest plantations, however, their suitability to restore preexisting habitats has been scarcely studied. This paper reports the effect of felling and forwarding of stone pine (Pinus pinea) plantations on soil disturbance and recovery of native xerophytic community typical of stabilised dunes in the Doñana Protected Area (southern Spain). Soil disturbance was assessed just after forwarding whereas recovery of plant assemblages was evaluated 27 months after the action by comparing species richness and diversity indices and Raunkiaer´s life functional types among four plot types: (i) plots with P. pinea that were not felled or forwarded, (ii) plots with P. pinea that were felled and forwarded, (iii) open areas without P. pinea affected by the forwarder and (iv) well-preserved open areas without P. pinea. Also, plant composition similarities between pairs of plots were evaluated with multivariate tests SIMPER and one-way ANOSIM. The forwarder provoked a shallow disturbance (litter and topsoil mixed) in areas of bare soil ≤ 20% and beside the Dorset heath, but deep disturbance (topsoil removed, subsoil exposed; and ruts) occurred in areas of bare soil>20% and on repeatedly used tracks. Non-forested areas affected by deep disturbance showed a significantly higher recovery of the xerophytic community than areas affected by shallow disturbance but previously occupied by a high cover of P. pinea. Our results provide novel evidence that a high cover of P. pinea slows down the recovery of native plant assemblages to a greater extent than the mere physical disturbance caused by the forwarder in areas without any pines. These results may guide future management actions aimed at enhancing biodiversity in natural areas affected by Pinus plantations and the adoption of responsible forestry practices.
Keywords: Management; Forest; Coastal; Logging; Shrub; Impact; Disturbance; Doñana
Introduction
Stone pine (Pinus pinea, hereinafter ‘Pinus’) is a xeric and thermophilic tree native to the Mediterranean region, which preferably colonises deep, loose, sandy and siliceous soils [1]. Nowadays, both spontaneous and cultivated Pinus forests occur throughout the Mediterranean basin, especially along coastal areas. The high landscape and historical value of these forests motivated its consideration as priority habitats (code 2270) by the European Council Directive 92/43/EEC. However, Pinus plantations outside their original forest areas may involve negative effects on pre-existing habitats such as over-exploitation of water, the reduction of light inside the forest mass, the accumulation of needles, an increased flammability or the decrease in seed germination [2-4].
In southern Spain, the first stone pine (Pinus pinea L., hereinafter ‘Pinus’) plantations date back to 1736 [5]. At the end of the 19th century and beginning of the 20th century, Pinus plantations aimed at stabilising ‘non-productive’ coastal sand dunes [1,6]. The rate of plantations reached 400 ha/year and over 190,000 ha of Pinus forests were created along the south-west coast of Spain [7]. In the Doñana Natural Area, Pinus plantations replaced more than 12,000 ha of alien Eucalyptus spp. that were planted since 1940 in response to the pressing need of raw materials (wood, paper pulp, essential oils) after the Spanish Civil War (1936–1939) [6,8]. The aim of Pinus plantations was not timber production but local people use these plantations for pine nuts production [9]. Pinus plantations aimed at fixing dunes and increasing the forest cover but took up natural pre-existing habitats such as sand dunes, riverbanks temporary wetlands and temperate Atlantic wet heaths with Dorset heath (Erica ciliaris). Thus, management of Pinus plantations towards biodiversity conservation poses a challenge of increasing importance [10]. However, reported actions concerning the effect of clearcutting and forwarding on biodiversity restoration are scarce [11,12] despite their importance to guide future planning in protected areas.
Particular techniques that have been traditionally used in timber plantations include felling and subsequent logging. Animals [13] or heavy machinery (e.g., forwarders and skidders) can be used for extract the logs. The latter is faster but potentially more aggressive on the soil surface, by causing soil disturbances and negative impacts on the biological community and the landscape [14-16]. In this sense, particular management actions in natural, protected areas may shed some light on the suitability of these techniques towards conservation of natural ecosystems. The magnitude of the disturbance caused by heavy machinery in the field depends on site conditions (soil type, soil humidity, the density of vegetation remains, etc.), the history and typology of the plantation, the typology of the pre-existing habitats, the method used to extract the logs, or the frequency of disturbance [17-21]. For example, tracked machines provoke a lower compaction than wheeled machines [22]. A slash layer and leaf litter may have a protective effect in sandy soils after repeated passes of a forwarder [18] and may reduce the formation of tyre ruts [23] whereas soil compaction may [24] or may not [25] be prevented after repeated passing by the machine. Despite the importance of physical disturbance and leaf litter in the outcome of actions aimed at ecological restoration, studies on the ecosystem response to particular logging practices are scarce [21,26]. Particularly, the reversibility of the impacts in terms of plant recovery after forwarding has been widely overlooked. With regards to removal of Pinus spp., a partial recovery of native vegetation one year after removal of maritime pine (Pinus pinaster) that encroached heathland areas in southern England was reported [27]. In this study, very few species recolonised the clear-cut areas [27]. Other authors found the vegetation that recovered three years after the clear-cut of stone pine plantations consisted mainly of re-sprouting residents but also annuals and alien species [11]. An increase of seeds from annual, non-forest species as well as seeds of alien species after the clear-cut of stone pine was reported [12]. These studies suggest that Pinus plantations may provoke long-lasting impacts typical of ecosystem engineers that may compromise the recovery of pre-existing habitats and their associated community [28]. Also, the risk of secondary colonisation of invasive plants in forwarded areas poses an additional challenge towards achieving the restoration goals.
This paper documents the results of clearcutting of 20.1 ha of Pinus plantations in the Doñana Natural Area, aimed at preserving pre-existing ecosystems such as Mediterranean xerophytic scrub and the Dorset heath. This study was conducted to find out the pattern of disturbance upon bare soil and plantation patches by forwarding of Pinus and their effects on recovery of xerophytic communities. This work was part of a European LIFE project (Conhabit Andalucía) aimed at enhancing priority habitats present in Natura 2000 sites along the Andalusian coast (southern Spain). Specifically, we assessed: (i) the impact of a high cover Pinus plantation in the Mediterranean xerophytic community with respect to open, unforested, well-preserved areas; (ii) the degree of soil disturbance caused by the forwarder; and (iii) the recovery of the xerophytic community 27 months after forwarding in plots with different degree of disturbance with and without Pinus cover. The results may help to improve responsible forestry practices, aimed at the management of old or disused forest plantations. Also, the present work will shed some light on vegetation recovery dynamics in forest gaps and exploited plantations.
Materials and Methods
Area of study
The managed Pinus plantations are located in the Doñana Natural Park (Huelva, SW Spain) (37°07’ N, 6°39’ W) (Figure 1), a protected area of ca. 68,200 ha included in the Doñana Natural Area (total protected area is ca. 120,000 ha) included in the Red Natura 2000 network of protected areas. This Natural Area constitutes one of the most significant wetland in Europe of special importance as a major stepping-stone for birds migrating between Africa and Europe [29]. It also includes one of the largest mobile dune systems in Europe. The Doñana Natural Area is home to a number of threatened, endemic and protected animals such as the Iberian lynx (Lynx pardinus), the Spanish imperial eagle (Aquila adalberti), the marbled teal (Marmaronetta angustirostris), the spider Donacosa merlini (Lycosidae), or the plants Adenocarpus gibbsianus (Fabaceae), Dianthus inoxianus (Caryophyllaceae), Linaria tursica (Scrophulariaceae), Gaudinia hispanica (Poaceae), etc. [30-32]. The climate is Mediterranean, with hot, dry summers and mild, wet winters [33]. Mean temperature and rainfall at Huelva meteorological station (27 km from the study area) for the period 1984-2010 are 18.2°C and 525 mm, respectively [34]. The soil is composed of eolian siliceous sands with a water table deeper than 1 m, with the surface horizon rich in carbonates (ca. 9%) that corresponds to the group ‘calcixeroll’ [35].
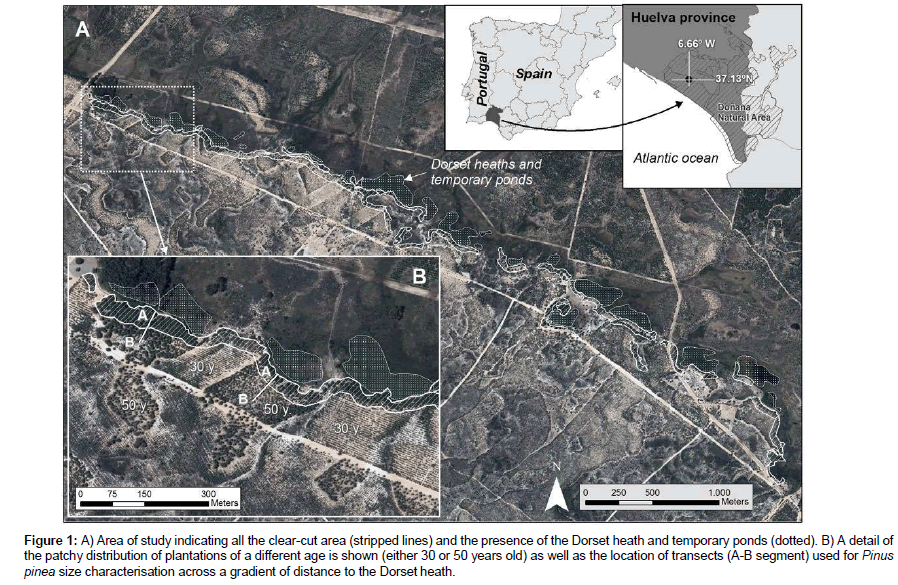
Figure 1: A) Area of study indicating all the clear-cut area (stripped lines) and the presence of the Dorset heath and temporary ponds (dotted). B) A detail of the patchy distribution of plantations of a different age is shown (either 30 or 50 years old) as well as the location of transects (A-B segment) used for Pinus pinea size characterisation across a gradient of distance to the Dorset heath.
The study area shows a patchy distribution of Pinus plantations made around 1968 and 1988 on stabilised dunes (Figure 1). This area constitutes an ecosystem boundary between Pinus forests rich in xerophytic scrub to the south and the Dorset heath to the north, locally known as Ribetehilos (Figure 1). A more detailed description can be found in Sousa et al. (2013). Pinus patches show a variable area (mean ± SD=5.2 ± 5.9 ha for the 1968 plantation, n=12; and 4.2 ± 3.1 ha for the 1988 plantation, n=7) (Figure 1). The 1968 plantation has a density of ca. 400 trees/ha whereas the 1988 plantation has a density of around 1000 trees/ha. Specifically, the xerophytic community studied is typical of stabilised dunes, locally known as ‘monte blanco’. The contiguous Dorset heath occurs in depressions with the water table being shallower than 1 m and common flooding in wet winters. Hygrophytic scrub is dominated by Dorset heath (Erica ciliaris) and dwarf gorse (Ulex minor) [36].
Characterisation of Pinus size within the plantation
Prior to clearcutting, the size (perimeter) of Pinus trees was measured throughout different patches of the older plantation (50 years old). First, perimeter of trees along two transects and four distance intervals (10–20 m, 30–40 m, 50–60 m and 70–80 m) from the Dorset heath was measured (Figures 1 and 2). Secondly, the effect of orientation was assessed in trees present in the border of the plantations (first 10 m). Measurements were taken from the trees facing towards the north-northeast, east, west and south, according to the patch arrangement in the study area. We measured n=46–61 trees for each category of distance and orientation of the border (N=451).
Felling and forwarding
Following the plantation characterisation and impact assessment on the xerophytic community (see below), the areas with the highest Pinus cover were selected for clearcutting. The clear-cut area included a narrow band (30 m) of Pinus plantations that runs along 6.7 km (total area=20.1 ha) (Figure 1). Felling was carried out with petrol portable chainsaws (Stihl® models 026 and 036; 48.7 and 61.6 c.c., respectively) in winter (December 2015), with the aim of minimising the impact on the accompanying flora and fauna and to promote the colonisation of spring annuals after forwarding. In-situ burning was not permitted by the Park authorities, therefore, a forwarder was used once the felling was completed (January-February, 2016). The forwarder used (John Deere®, model 1710D) is 10.85 m long, and 3.9 m high (not loaded) and weighs 19,000 kg (tare), with the ability to carry a maximum load of wood of 17,000 kg on a loader with compression capacity (Figure SI1). All the logs and branches removed were piled up in six collection points, near pre-existing tracks and were later crushed for biomass. In order to avoid widespread impacts on the soil surface, existing tracks and fire-breakers were used, going over the same tracks whenever possible.
Effects of the forwarder on the soil surface
Once the felling and forwarding of Pinus was completed, the magnitude of disturbance of the soil surface was assessed, based on the MacMahon classification [15,37]. During analysis of disturbance in the field, areas that showed a similar magnitude of disturbance were georeferenced using a GPS (Garmin® model Etrex 10). We used the geodetic reference system ETRS89 (European Terrestrial Reference System 1989) and UTM projection zone 30 according to European standards. The results were processed using the ArcGIS® v. 10.2.2 software. Polygon areas were calculated using the ‘calculate geometry’ tool. Thus, the working area was divided into 69 polygons that summed a total area of 20.54 ha. This area is somewhat higher than the clearcut area (20.1 ha) because we included in the analysis tyre tracks used during forwarding. Each polygon was assigned the following parameters: (i) a disturbance category, (ii) the age of the forest plantation (30 or 50 years old for the 1988 and 1968 plantation, respectively), (iii) the percentage of bare soil; and (iv) the distance to the nearest Dorset heath. The distribution of each type of polygon was: 30-year plantation (n=18; 7.42 ha), 50-year plantation (n=31; 8.52 ha), roads and tyre track areas (n=15; 3.78 ha), and polygons without plantation, with natural vegetation (n=5; 0.81 ha).
Recovery of the xerophytic community typical of stabilised dunes
Twenty seven months after forwarding, the plant community composition was analysed. Four different plot types were considered (Figures 2 and 3; see appearance in Figure SI2): (a) 50 years old Pinus patches, with a cover=100%, that were not clear-cut or forwarded (hereinafter ‘Pinus plots’); (b) 50 years old Pinus patches, with a cover=100%, that were clear-cut and forwarded (hereinafter ‘Pinus- forwarded plots’); (c) open areas without Pinus that were affected by deep disturbance by the forwarder (hereinafter ‘open, forwarded plots’); and (d) open areas without Pinus with well-preserved, natural vegetation typical of stabilised dunes that were not affected by the forwarder (hereinafter ‘open, well-preserved plots’). These plots were used as the reference state to which ‘Pinus-forwarded plots’ and ‘open, forwarded plots’ should reach. Thus, comparison between ‘Pinus plots’ and ‘open, well-preserved plots’ reported information on the impact provoked by Pinus pinea on native plant assemblages (‘A’ in Figure 3). However, direct comparison between ‘Pinus plots’ and ‘open, well- preserved plots’ may not indicate causality. It is therefore necessary to analyse the plant community response after removal experiments [38]. Therefore, comparison between ‘Pinus-forwarded plots’ and ‘open, well-preserved plots’ provided additional information on the effect of Pinus and the recovery of the xerophytic community after Pinus logging (‘B’ in Figure 3). Comparison between ‘open, forwarded plots’ and ‘open, well-preserved plots’ provided information on the plant recovery after deep disturbance caused by the forwarder in open areas without Pinus (C in Figure 3). Finally, comparison between A and C provided information on the relative importance and reversibility of impacts of Pinus plantations and physical disturbance (caused by the forwarder) on the xerophytic community. Thus, the use of ‘open, well-preserved plots’ in pairwise comparisons allowed us to distinguish changes caused by Pinus removal, since observing treatment plots over time may not allow differentiation of treatment effects from changes due to natural fluxes [39].
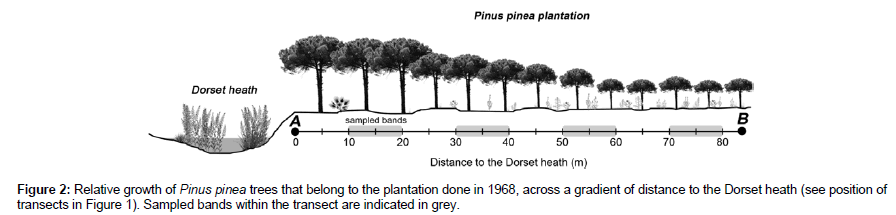
Figure 2: Relative growth of Pinus pinea trees that belong to the plantation done in 1968, across a gradient of distance to the Dorset heath (see position of transects in Figure 1). Sampled bands within the transect are indicated in grey.
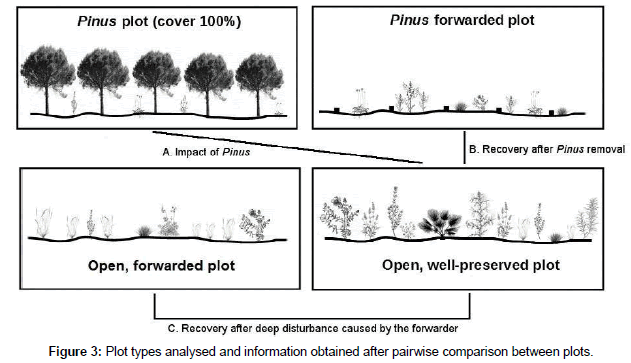
Figure 3: Plot types analysed and information obtained after pairwise comparison between plots.
The different plot types showed similar characteristics (slope and soil type). The presence of plants in 70 quadrats of 1 m2 was noted for each plot (N=280). Samplings were done in spring (April, 2018) to record spring ephemerals and woody, perennial plants. Species with occurrences ≤ 3% were excluded from analyses. Plant determinations and nomenclature followed Flora Iberica [40] except for families Gramineae and Compositae (not included in Flora Iberica) for which a different guide was used [41]. Species were classified as native or alien to the Iberian Flora [40], and categorised into Raunkiaer´s life functional types [42] and typical habitat (generalist and ruderal species vs. species typical of stabilised dunes). Life forms were clustered into five main types: (1) Phanerophytes: trees, shrubs and vines; (2) Chamaephytes: small bushes and herbs with perennial buds within 25 cm from the soil surface; (3) Hemicryptophytes: perennial herbaceous plants with buds at the soil surface; (4) Geophytes: perennial herbaceous plants with dormant parts below the soil surface; and (5) Therophytes: annual plants.
Statistical analysis
The non-parametric Kruskal-Wallis test was applied to compare the medians of trunk perimeters for the Pinus trees situated in different orientations and for the trees situated at different distances from the Dorset heath. Next, pairwise ranking was used with data from the different categories of distance using a Mann-Whitney U test. We performed a principal component analysis (PCA) to assess the percentage of variance explained by the following variables: % of bare ground, age of plantation, and distance to the nearest Dorset heath. PCA is a multivariate procedure that transforms a number of (possibly) correlated variables into a smaller number of uncorrelated variables called principal components. The principal components are linear combinations of the original variables weighted by their contribution to explaining the variance in a particular orthogonal dimension. The corresponding eigenvalues measure the amount of information explained by the principal components [43]. For assessing the impact of Pinus on the xerophytic community typical of stabilised dunes as well as community recovery after forwarding or disturbance, we used the community composition data obtained in the plots. The species richness, the Brillouin´s diversity index (HB), the dominance index (D) and the Buzas and Gibson´s evenness index were calculated [43]. HB poses some advantages with respect to the most popular diversity index (Shannon´s diversity index, H´) [44]. However, the former indexes value all species equally and do not evidence shifts in composition. For example, an increase of species richness after removal of the target species may not mean a recovery of the native community typical of the well-preserved state but just an increase of therophytes typical of degraded conditions [38]. For this reason, multivariate tests SIMPER and one-way ANOSIM were applied to in order to interpret plant community response to clearcutting based on the identity of all plant species present in the plots. The software Past3 was used [43]. The SIMPER test calculates the percentage of dissimilarity between pairs of plots, as well as the contribution of each species to overall dissimilarity. ANOSIM is a non-parametric test that assesses the overall significance of the difference between predefined groups (plot types) by reporting significance (p) and R values. R statistic compares average similarities within groups and between groups. R theoretically varies between -1 and 1. R values close to 1 indicate high dissimilarity between groups while close to 0 values indicate no differences in community composition between plot types [45]. Both analyses were based on the Bray-Curtis similarity measure. Differences were considered significant when p<0.05.
Results
Characterisation of Pinus size within the plantation
The Pinus trees closest to the Dorset heath showed a significantly (p<0.0001, U Mann-Whitney test) bigger size (median perimeter=1.66 m at 10–20 m) than trees of the same age planted further away from the Dorset heath (Figure 4A). The perimeter decreased with increasing distances from the Dorset heath, with significant differences between 10–20 m and all the other distances and between 30–40 m and the other distances (Mann-Whitney U test). Beyond 30–40 m from the Dorset heath, the perimeters did not show significant differences. The orientation of Pinus throughout the plantation patches also caused significant differences (p<0.0001, Kruskal-Wallis test) in tree size (Figure 4B), according to the following decreasing order (the median perimeter is indicated): north-northeast in the vicinity of the Dorset heath (1.66 m)>east (0.90 m)>north-northeast away from the Dorset heath (0.88 m)>west (0.84 m)>south (0.78 m). The differences were significant in the following cases: north-northeast in the vicinity of the Dorset heath with respect to any other orientation (p<0.0001, Mann-Whitney U test), north- northeast away from the Dorset heath vs. south (p=0.0006, Mann-Whitney U test) and east vs. south (p=0.0009, Mann-Whitney U test).
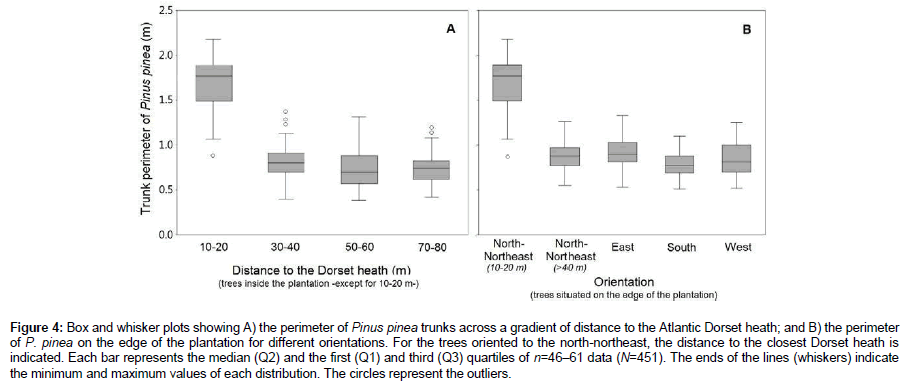
Figure 4: Box and whisker plots showing A) the perimeter of Pinus pinea trunks across a gradient of distance to the Atlantic Dorset heath; and B) the perimeter of P. pinea on the edge of the plantation for different orientations. For the trees oriented to the north-northeast, the distance to the closest Dorset heath is indicated. Each bar represents the median (Q2) and the first (Q1) and third (Q3) quartiles of n=46–61 data (N=451). The ends of the lines (whiskers) indicate the minimum and maximum values of each distribution. The circles represent the outliers.
Effects of the forwarder on the soil surface
Two main categories of disturbance, i.e., shallow (litter and topsoil mixed) and deep (topsoil removed, subsoil exposed; and tyre ruts) were observed. In short, the proportion of area affected by a deep disturbance after felling and forwarding was 64% (13.2 ha) compared with the 36% (7.3 ha) affected by shallow disturbance. Deep disturbances were identified in areas with a percentage of bare soil>30% before the forwarding and relatively far away (median distance=33.5 m) from the Dorset heath. Shallow disturbance was identified near the Dorset heath (median distance=21 m) and on soils with percentages of bare soil<20% (Figure 5). Deep disturbance was also identified in areas repeatedly transited by the forwarder that lacked a forest cover. PCA analysis revealed that soil disturbance was mainly (95% variance) explained by the distance to the closest Dorset heath and the pre-existing percentage of bare ground (Table 1; Figures 5 and 6). In contrast, the age of the plantation (represented in PCA component 3) poorly affected the magnitude of soil disturbance caused by the forwarder (4.75% variance, Table 1).
Table 1: Results of the PCA, showing the % of variance explained by each habitat variable on the magnitude of soil disturbance.
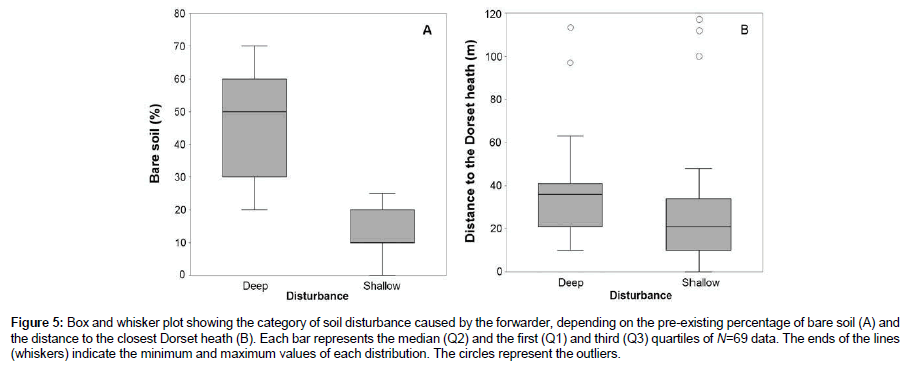
Figure 5: Box and whisker plot showing the category of soil disturbance caused by the forwarder, depending on the pre-existing percentage of bare soil (A) and the distance to the closest Dorset heath (B). Each bar represents the median (Q2) and the first (Q1) and third (Q3) quartiles of N=69 data. The ends of the lines (whiskers) indicate the minimum and maximum values of each distribution. The circles represent the outliers.
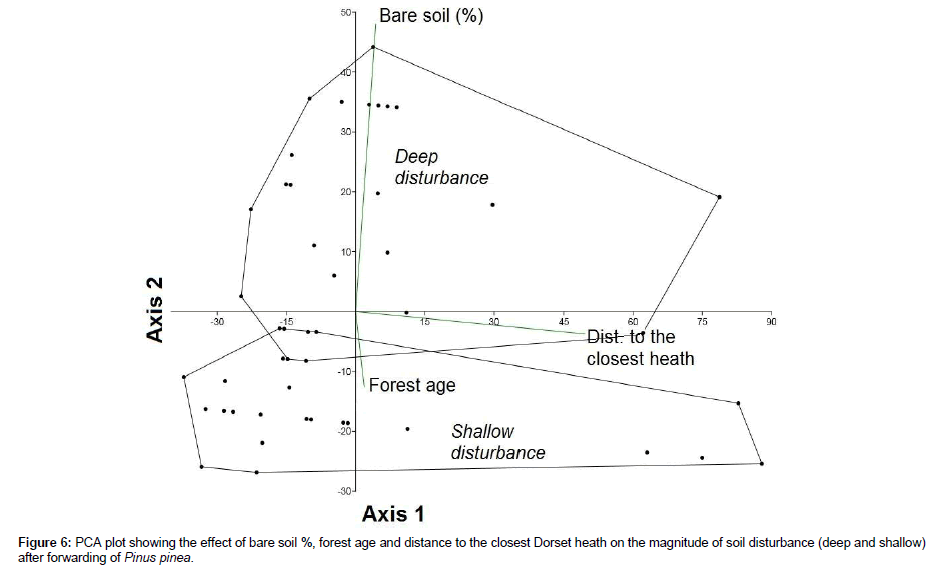
Figure 6: PCA plot showing the effect of bare soil %, forest age and distance to the closest Dorset heath on the magnitude of soil disturbance (deep and shallow) after forwarding of Pinus pinea.
Impact of Pinus on the xerophytic community and recovery after forwarding
‘Pinus plots’ showed the lowest richness (S=18) and Brillouin´s diversity index (HB=2.07) among the four plot types studied (Table 2). The relatively high value of the dominance index (D=0.17) was explained by the relative higher abundance of geophytes such as Aetheorhiza bulbosa (mean occurrence=93%) and other ruderal, generalist species than in ‘open, well-preserved plots’ (Table 3, Figure 7A). Accordingly, SIMPER analysis revealed the greatest dissimilarities (90.8%) in plant composition between ‘Pinus plots’ and ‘open, well- preserved plots’ (Table 3).
Table 2: Richness, diversity, evenness and diversity indexes of the four plot types analysed.
Table 3: Results of the pairwise Simper and Anosim tests between plots.
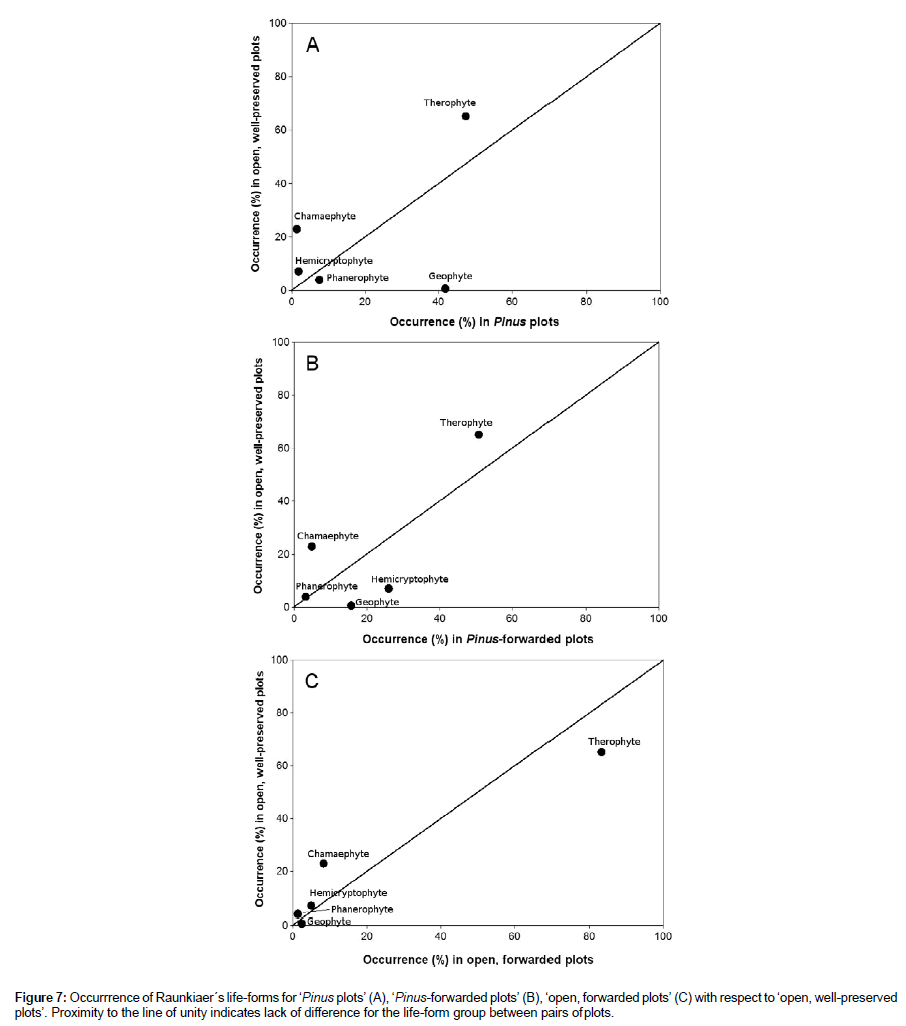
Figure 7: Occurrrence of Raunkiaer´s life-forms for ‘Pinus plots’ (A), ‘Pinus-forwarded plots’ (B), ‘open, forwarded plots’ (C) with respect to ‘open, well-preserved plots’. Proximity to the line of unity indicates lack of difference for the life-form group between pairs of plots.
Twenty-seven months after felling and forwarding, the xerophytic community showed a variable recovery that depended on the previous Pinus cover and the magnitude of soil disturbance. Species richness and Brillouin´s diversity index increased both in ‘Pinus-forwarded’ and ‘open, forwarded plots’ with respect to ‘Pinus plots’. The maximum richness (S=27) and diversity (HB=2.66) values were measured in ‘Pinus-forwarded plots’ (Table 2), where a massive colonisation of generalist terophytes such as African mustard (Brassica tournefortii), soft brome (Bromus hordeaceus), and Senecio sp. occurred. These values were even greater than in ‘open, well-preserved plots’ (S=24; HB=2.64). Instead, ‘open, forwarded plots’ were colonised by therophytes typical of poor nutrient sandy soils (e.g., Arenaria algarbiensis, Corynephorus fasciculatus, Malcolmia triloba and Vulpia fontquerana) (Table 4). These annual plants were also represented in ‘open, well-preserved plots’ but not in ‘Pinus-forwarded plots’ (Figure 8). As a consequence, ‘open, forwarded plots’ showed the lowest dissimilarity (39.6%) with respect to ‘open, well-preserved plots’. In contrast, dissimilarity between ‘Pinus-forwarded plots’ and ‘open, well-preserved plots’ was rather high (84%) (Table 3). Figure 7 represents the percent occurrence of the different Raunkier´s functional groups of ‘Pinus plots’, ‘Pinus- forwarded plots’ and ‘open, forwarded plots’ with respect to ‘open, well-preserved plots’ (Figure 7). Only two of the five functional groups showed a significant increase after Pinus removal (geophytes and hemicryptophytes) (Figure 7B). However, occurrence of therophytes was greater in ‘open, forwarded plots’ than in ‘open, well-preserved plots’ (Figure 7C). Occurrence of chamaephytes was lower in ‘Pinus- forwarded plots’ and ‘open, forwarded plots’ than in ‘open, well- preserved plots’ (Figure 7B-C).
Table 4: Mean occurrence (%) of plant species in the different plots analysed. Each value corresponds to the average cover of 70 quadrats per plot (N=280). Taxa with occurrences ≤ 3% were excluded from analyses. Taxa with occurrences ≥ 20% are highlighted in bold.
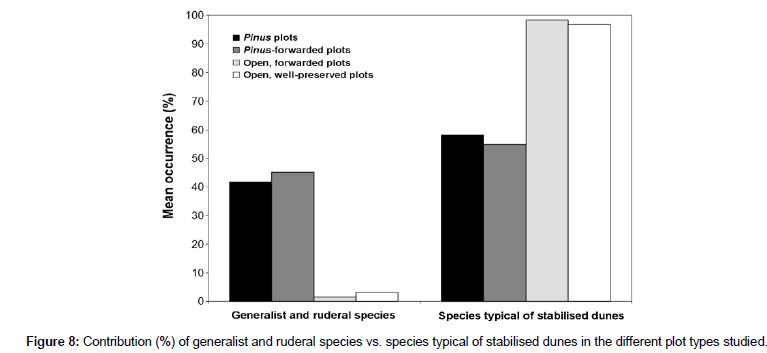
Figure 8: Contribution (%) of generalist and ruderal species vs. species typical of stabilised dunes in the different plot types studied.
The ANOSIM test revealed significant differences (p ≤ 0.001) in the xerophytic community globally and in all pairwise comparisons between plots. This means that either ‘Pinus-forwarded plots’ or ‘open, forwarded plots’ still differs from ‘open, well-preserved plots’. The R statistic shows values equal to one or very close to 1 (Table 3), also showing that the xerophytic community between plots is still very different.
Discussion
In order to reverse the global loss of natural habitats, the conversion of old plantations towards biodiversity conservation is becoming a growing practice [10]. Felling and forwarding of Pinus in the present work aimed at recovering the number and abundance of species characteristic to the original ecosystems [46]. However, Pinus forests are also recognised as a priority habitat and in the Doñana Natural Area overlap other priority habitats such as the Dorset heath (code 4020), Atlantic decalcified fixed dunes (Calluno-Ulicetea) (code 2150) and Malcolmietalia dune grasslands (code 2230). The coincidence in space of four priority habitats posed a challenge during the planning stage that requested to define conservation priorities. The sharp decrease of the Dorset heath observed in the study area since the XVII century [47] tipped the balance towards the conservation of this habitat and fixed dunes over high cover Pinus plantations.
The high dissimilarity (90.8%) between ‘Pinus plots’ and ‘open, well-preserved plots’ suggests that Pinus plantations may outcompete the Mediterranean xerophytic scrub when Pinus trees reach a high cover (100%). Our results agree with the diversity loss reported in plantations replacing natural shrublands [48]. Also, a high canopy cover in Pinus spp. forests from coastal Italy was associated with generalist and alien species, whereas lower canopy cover supported herbaceous and shrub species typical of natural dunal succession [49]. Our results also agree with the significant decrease of woody species richness and overall shrub cover reported in heathlands of the Strait of Gibraltar invaded by maritime pine (Pinus pinaster) [50]. Accordingly, felling and forwarding of high cover Pinus patches is proposed as a general recommendation to improve the biodiversity of the native shrubland, particularly in coastal sand dunes.
Our results showed that the outcome of restoration may depend on particular site conditions such as the distribution of wetlands (here represented by the Dorset heath), the Pinus cover and the percentage of bare soil. The fact that the age of the plantation explained a small percentage of the variance was related to small-scale (tens of meters) differences of Pinus growth within a plantation of the same age. The size differences of Pinus trees throughout the plantation affected the magnitude of soil disturbance, in accordance with [28]. In practice, the higher growth of Pinus motivated a higher cover (measured as the tree crown projection) because crowns joined together. The higher growth is responsible of a greater accumulation of needles beneath the Pinus trees and a shallow soil disturbance on the areas closest to the Dorset heath than in further away distances. Despite the native origin of Pinus, our results agree with previous reports of higher Pinus growth in areas close to freshwater bodies [51], since the root systems of Pinus can extend to reach the groundwater level [52]. Pinus can have fairly developed root systems, essential for adapting to the summer droughts of the Mediterranean region. The architecture of Pinus roots consists basically of a taproot with several lateral roots leading off it [9]. These lateral roots are divided (sometimes in a ‘T’, as new rootlets) to form a complex multi-layered radical system [53]. The surface area of the Pinus radical system can reach 8–50 times the surface area of the projection of the tree crown [54]. The depth of the taproots ranged from 0.88 to 1.80 m in Pinus trees from Ravenna (Italy) [55,56] and from 0.70 to 1.55 m in the south of France [55]. Regarding the lateral roots, lengths have been measured ranging from 2.4 to 37.8 m for trees of 24 and 128 years of age, respectively [57]. These lengths can vary with the nature of the soil, developing better in sandy soils (like in the area of study) than in clayey soil [9]. Although we do not have specific data on the extension of the roots of the pine trees in the area studied, the data published suggests that the Pinus roots have potential to capture water that nourishes the heath, at least in plantations situated up to 30–40 m away from the Dorset heath (especially in the first 20 m). This fact was confirmed by particular in situ observations in the study area (Figure SI3).
The generation of ruts and rutting, particularly on repeatedly used tracks and pre-existing fire breaks where the percentage of bare soil was ca. 90% agrees with [18], who found a greater degree of protection of the layer of organic matter (density of leaf litter up to 20 kg m-2) in sandy soils after five years of constant traffic. The layer of leaf litter reduces the formation of ruts [23] although may not prevent compacting or other related disturbances (porosity) after repeated passing by the machine [25]. Furthermore, in areas with a high density of felled trees, the forwarder (with an arm reaching a maximum distance of 8.5 m) can remove more biomass by maintaining its position, reducing the level of disturbance. On the other hand, when the trees or branches to be removed are more disperse, the forwarder needs to constantly reposition itself and the magnitude of disturbance is greater.
Our results showed that the removal of Pinus did not allow a recovery of the xerophytic community of stabilised dunes (dissimilarity=84% after 27 months) after 27 months but induced the colonisation of ruderal terophytes suggesting that the high cover of Pinus modified the habitat [4,28]. Therefore, the complete recovery of plant assemblages in high cover Pinus patches may need a longer period, as found after ecological restoration of forestryâÂÂdrained peatlands (5-10 years) [46]. The recovery observed in ‘Pinus-forwarded plots’ was in fact much lower (dissimilarity=90.8%) than in open areas that underwent a deep disturbance by the forwarder (dissimilarity=39.6%). The increase in annual plants typical of stabilised dunes after forwarding and the lower contribution of long life cycle chamaephytes suggests that the plant community is still in an early successional stage. This result is coherent with the relative short period elapsed since the disturbance and agrees with previous reports [12,13]. Strikingly, physical disturbance provoked by the forwarder stimulated the development of meadows typical of the habitat of community interest (code 2230) ‘Malclomietalia dune grasslands’, which included some threatened and/or protected species such as Vulpia fontquerana [30]. Therefore, Pinus plantations would thus have a more lasting effect than the mere physical disturbance caused by the forwarder. This suggests that sandy soils have a relatively high recovery potential to physical disturbance compared with other types of environments [58]. This positive result may be also explained because the treated areas are adjacent to source habitats [59]. It also suggests that the xerophytic plants recover better in areas disturbed by the repeated crossing of the forwarder than in areas previously occupied by a high cover of Pinus. Finally, the absence of a secondary colonisation by alien species reported in other studies [12,13,53] may be explained by the rather long distance of the study area to the closest agricultural and urban areas (13 km) that may act as propagule source of undesirable species.
Conclusion
The forwarder mainly affected the areas with a percentage of bare soil>20%. The impact of the forwarder on the xerophytic plant community typical of stabilised dunes showed promising signs of reversibility in areas in which the forwarder caused deep disturbance compared with others with a shallower disturbance but with a high Pinus cover. Our results suggest that a high cover of Pinus have a more lasting effect than the mere physical disturbance caused by the passing of the forwarder in areas without any pines. Our findings may serve as a guide for future management and planning of old, disused Pinus plantations in natural areas aimed at prioritising biodiversity conservation.
Acknowledgements
This project has been financed with the LIFE CONHABIT ANDALUCÍA PROJECT (LIFE13/NAT/ES/000586), coordinated by the Regional Ministry for the Environment and Land Planning of the Regional Government of Andalusia, and it is 60% co-financed via the Life+ programme, an EU financial instrument for the environment.
References
- Martínez F, Montero G (2004) The Pinus pinea L. woodlands along the coast of South-Western Spain: data for a new geobotanical interpretation. Plant Ecol 175: 1-18.
- Tomei PE, Bertacci A, Sani A Consiglio M (2004). La vegetazione della Tenuta di San Rossore. Note esplicative della carta della vegetazione di San Rossore 1:10.000.
- Ormeño E, Céspedes B, Sánchez IA, Velasco-García A, Moreno JM, et al. (2009). The relationship between terpenes and flammability of leaf litter. For Ecol Manage 257: 471-482.
- Valera-Burgos J, Díaz-Barradas MC, Zunzunegui M (2012) Effects of Pinus pinea litter on seed germination and seedling performance of three Mediterranean shrub species. Plant Growth Regul 66: 285-292.
- Granados M, Martín A, García-Novo F (1983) Introducción del P. pinea en el Parque Nacional de Doñana. Actas del Seminario sobre Reservas de la Biosfera, La Rábida, Huelva.
- Sousa A, García-Murillo P (2001) Can place names be used as indicators of landscape changes? Application to the Doñana Natural Park (Spain). Landscape Ecol 16: 391-406.
- Martínez F, Montero G, Ruiz-Peinado R, Cañellas I, Candela JA (2004) Geobotánica e historia de los pinares, In: Montero G, Candela JA, Rodríguez A (Eds.), El pino piñonero (Pinus pinea L.) en Andalucía. Consejería de Medio Ambiente, Junta de Andalucía, pp. 49-112.
- Custodio E, Manzano M, Montes C (2009). Las aguas subterráneas en Doñana: aspectos ecológicos y sociales. Sevilla: Agencia Andaluza del Agua.
- Montero G, Candela JA, Rodríguez A (2004). El pino piñonero (Pinus pinea L.) en Andalucía. Consejería de Medio Ambiente, Junta de Andalucía, Sevilla.
- Chazdon RL (2008). Beyond deforestation: restoring forests and ecosystem services on degraded lands. Science 320: 1458-1460.
- Arduini I, Ercoli L (2012) Recovery of understorey vegetation in clear-cut stone pine (Pinus pinea) plantations. Plant Biosyst 146: 244-258.
- Arduini I, Orlandi C, Mariotti M, Masoni A (2013) Seed banks and forest recruitment after disturbance: the composition and abundance of seed after the clear-cut of stone pine (Pinus pinea L.) plantations. Pol J Ecol 61: 457-470.
- Shrestha SP, Lanford BL, Rummer RB, Dubois M (2005) Utilization and cost of log production from animal logging operations. Intl J For Eng 16: 167-180.
- Swanson FJ, Dyrness CT (1975) Impact of clear-cutting and road construction on soil erosion by landslides in the western Cascade Range, Oregon. Geology 3: 393-396.
- Gondard H, Romane F, Aronson J, Shater Z (2003) Impact of soil surface disturbances on functional group diversity after clear-cutting in Aleppo pine (Pinus halepensis) forests in southern France. Forest Ecol Manag 180: 165-174.
- Marchi E, Picchio R, Spinelli R, Verani S, Venanzi R, et al. (2014) Environmental impact assessment of different logging methods in pine forests thinning. Ecol Eng 70: 429-436.
- Keenan RJ, Kimmins JP (1993) The ecological effects of clear-cutting. Environmental Rev 1: 121-144.
- McDonald TP, Seixas F (1997) Effect of slash on forwarder soil compaction. J For Eng 8: 15-26.
- Lindenmayer DB, Noss RF (2006) Salvage Logging, ecosystem processes, and biodiversity conservation. Conserv Biol 20: 949-958.
- Bunce RGH, Wood CM, Smart SM, Oakley R, Browning G, et al. (2014) The landscape ecological impact of afforestation on the British Uplands and some initiatives to restore native woodland cover. J Landscape Ecol 7: 5-24.
- Kleibl M, KlvaÄÂ R, Lombardini C, Porhaly J, Spinelli R (2014) Soil compaction and recovery after mechanized final felling of Italian coastal pine plantations. Croat J For Eng 35: 63-71.
- Allman M, Jankovský M, Messingerová V, Allmanová Z, FerenÄÂík M (2015) Soil compaction of various Central European forest soils caused by traffic of forestry machines with various chassis. For Syst 24: e038.
- Wronski EB (1980) Logging trials near Tumut. Logger April/May: 10-14.
- McMahon S, Evanson T (1995) The effect of slash cover in reducing soil compaction resulting from vehicle passage. Logging Industry Research Organisation, Project Report 19. Logging Industry Researc Organisation, New Zealand.
- Jakobsen BF, Moore GA (1981) Effects of two types of skidders and of a slash cover on soil compaction by logging of mountain ash. Aust For Res 11: 247-255.
- Kern CC, Palik BJ, Strong TF (2006) Ground-layer plant community responses to even-age and uneven-age silvicultural treatments in Wisconsin northern hardwood forests. Forest Ecol Manag 230: 162-170.
- Liley D (2005) Mechanical clearance of maritime pine Pinus pinaster using a shear-head timber processor at Barnsfield, Dorset, England. Conserv Evidence 2: 105-106.
- Ohlson M, Okland RH, Nordbakken JF, Dahlberg B (2001) Fatal interactions between Scots pine and Sphagnum mosses in bog ecosystems. Oikos 94: 425-432.
- García Novo F, Marín-Cabrera C (2006) Doñana: Water and Biosphere, Doñana 2005 Project. Guadalquivir Hydrologic Basin Authority, Spanish Ministry of the Environment, Madrid, Spain.
- Cabezudo B, Talavera S, Blanca G, Salazar C, Cueto M, et al. (2005) Lista roja de la flora vascular de Andalucía. Consejería de Medio Ambiente. Junta de Andalucía, spain.
- Moreno JC (coord.) (2008) Lista Roja 2008 de la flora vascular española. Madrid, Spain.
- Sánchez I (2008) Donacosa merlini Alderweireldt y Jocqué, 1991. In: Libro Rojo de los Invertebrados de Andalucía. Consejería de Medio Ambiente, Junta de Andalucía, Sevilla, Spain.
- Kottek M, Grieser J, Beck Ch, Rudolf B, Rubel F (2006) World Map of the Köppen-Geiger climate classification updated. Meteorol Z 15: 259-263.
- Aemet (2018) Valores climatológicos normales, Andalucía, Huelva, Ronda Este. Spain.
- Clemente L, García-Fernández LV, Siljestrom P (1998) Los suelos del Parque Nacional de Doñana. Instituto de Recursos Naturales y Agrobiología de Sevilla, Spain.
- Díaz-Barradas MC, Zunzunegui M, Tirado R, Ain-Lhout F, García-Novo F (1999) Plant functional types and ecosystem function in Mediterranean shrubland. J Veg Sci 10: 709-716.
- McMahon S (1995) A survey method for assessing site disturbance. Project Report 54. Logging Industry Researc Organisation, New Zealand.
- Andreu J, Manzano-Piedras E, Bartomeus I, Dana ED, Vilà M (2010) Vegetation response after removal of the invasive Carpobrotus hybrid complex in Andalucía, Spain. Ecol Restor 28: 440-448.
- Swab RM, Zhang L, Mitsch WJ (2008) Effect of hydrologic restoration and Lonicera maackii removal on herbaceous understory vegetation in a bottomland hardwood forest. Restor Ecol 16: 453-463.
- Castroviejo S (coord) (1986-2012) Flora iberica 1-8, 10-15, 17-18, 21. Real Jardín Botánico, CSIC, Madrid, Spain.
- Valdés B, Talavera S, Fernández-Galiano E (1987) Flora Vascular de Andalucía Occidental, vol. 3. Ketres editora S.A., Barcelona, Spain.
- Smith WG (1913) Raunkiaer´s “life-forms” and statistical methods. J Ecol 1: 16-26.
- Hammer Ø (2001) PAST PAleontological STatistics Version 3.20. Reference manual. Natural History Museum, University of Oslo, Norway.
- Magurran AE (2004) Measuring biological diversity. Blackwell Publishing Company, Victoria, Australia.
- Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation. Plymouth: Plymouth Marine Laboratory. Primer software, UK.
- Haapalehto T, Juutinen R, Kareksela S, Kuitunen M, Tahvanainen T, et al. (2017) Recovery of plant communities after ecological restoration of forestry-drained peatlands. Ecol Evol 7: 7848-7858.
- Sousa A, Morales J, García-Barrón L, García-Murillo P (2013) Changes in the Erica ciliaris Loefl. ex L. peat bogs of southwestern Europe from the 17th to the 20th centuries AD. Holocene 23: 255-269.
- Bremer LL, Farley KA (2010) Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness. Biodivers Conserv 19: 3893-3915.
- Bonari G, Acosta ATR, Angiolini C (2017) Mediterranean coastal pine forest stands: Understorey distinctiveness or not? Forest Ecol Manag 391: 19-28.
- Andrés C, Ojeda F (2002) Effects of afforestation with pines on woody plant diversity of Mediterranean heathlands in southern Spain. Biodivers Conserv 11: 1511-1520.
- Gardner LR, Michener WK, Williams TM, Blood ER, Kjerve B, et al. (1992) Disturbance effects of hurricane Hugo on a pristine coastal landscape: North Inlet, South Carolina, USA. Neth J Sea Res 30: 249-263.
- Antonellini M, Mollema PN (2010) Impact of groundwater salinity on vegetation species richness in the coastal pine forests and wetlands of Ravenna, Italy. Ecol Eng 36: 1201-1211.
- Profili V (1993) Analisi morfologiche degli apparati radicali di pino domestico (Pinus pinea L.) nella pineta di Alberese. Tesi di Laurea. Istituto di Selvicoltura. Università degli Studi di Firenze.
- Cabanettes A, Rapp M (1978) Biomasse, minéralomasse et productivité d'un écosystème à Pins pignons (Pinus pinea L.) du littoral méditerranéen. I. - Biomasse. Acta oecologica. 13: 271-286.
- Padula M (1968) Ricerche sulle condizioni ecologiche dei boschidi San Vitale e di Classe (Ravenna), ai fini del loromiglioramento colturale, con saggi di esame degli apparatiradicali di Pinus e Quercus. Annali dell´Accademia Italiana di Scienze Forestali. Firenze XVII: 173-246.
- Cabanettes A (1979) Croissance, biomasse et productivité de Pinus pinea L. en Petite Camargue. Thèse 3ème Cycle U.S.T.L. Montpellier.
- Agrimi M, Ciancio O (1993) Le pin pignon (Pinus pineaL.), FAO, Rome, Itlay.
- Hesp PA, Martínez ML (2007) Disturbance processes and dynamics in coastal dunes. In: Plant disturbance ecology, The process and the response. Elsevier, New York, USA.
- Flinn KM, Vellend M (2005) Recovery of forest plant communities in post-agricultural landscapes. Front Ecol Environ 3: 243-250.