Research Article, J Biochem Physiol Vol: 1 Issue: 2
HPLC Profiles of Onion Fructooligosaccharides and Inulin and their Prebiotic Effects on Modulating Key Markers of Colon Function, Calcium Metabolism and Bone Mass in Rat Model
Hoda Mabrok, Magda Soliman, Mahmoud Mohammad and Laila Hussein*
Department of Human Nutrition, National research Center, Dokki-Gizah, Egypt
*Corresponding Author : Laila Hussein
Department of Human Nutrition, National research Center, Dokki-Gizah, 12311, Egypt
Tel: 01006957715
E-mail: dr.lailahussein@yahoo.com
Received: March 28, 2018 Accepted: April 12, 2018 Published: April 19, 2018
Citation: Mabrok H, Soliman M, Mohammad M, Hussein L (2018) HPLC Profiles of Onion Fructooligosaccharides and Inulin and their Prebiotic Effects on Modulating Key Markers of Colon Function, Calcium Metabolism and Bone Mass in Rat Model. J Biochem Physiol 1:1.
Abstract
The objective of this study was to purify and characterize the fructooligosaccharides (FOS) and inulin (INU) contents in local onion varieties. Vigorous extraction of the onion with hot water recovered over 93% of the initial non digestible carbohydrates (NDC). HPLC separation and quantitation showed that total FOS accounted for 20% and INU for 16% of the dry onion NDC extract. The prebiotic effect of onion NDC was tested by incorporating the freeze dried onion NDC at 13% in semisynthetic diet fed to Sprague Dawley rats for 5 weeks. Commercial FOS and INU were run in parallel and served as references. Key markers of colon function included measuring enlargement in colon weight as criteria of bifidogenic effect and colonization, activities of the caecal glycolytic enzymes, lowering in the activity of the glucuronidase and lowering of caecal pH value. The effect of dietary onion NDC on different aspects of calcium metabolism and bone mass was studied as well. The consumption of dietary onion NDC, FOS and INU for 5 weeks was associated with significant shifting in the fecal pH value towards the acidic direction, increase in the caecal weight due to colonization with beneficial bacteria and significant increases in the activities of caecal α- and β-glucosidases and α- and β- galactosidases compared with respective results obtained with the control group. The enzyme activity of the caecal glucuronidase was reduced compared with the respective control group. Parameters of calcium metabolism included % absorption, balance, retention, femur and tibia calcium contents were significantly improved compared to the respective control group. Conclusion: Adaptation to a diet containing prebiotic from onion led to positive physiological changes in the gut biomarkers of microbial activity This study provides direct evidence that onion (NDC) can act as an orally active agent for preserving bone mass in a growing rat model (aged 3-6 months),which has great implication on good health. Accurate quantitation of FOS and INU will allow the development of functional food formulations in human diets.
Keywords: Egyptian onion varieties; Fructooligosaccharides; Inulin; HPLC; Rat trial; Colon glycolytic enzymes; Calcium absorption and retention; Femur mineral density and strength
Introduction
After starch, fructans are the most abundant nonstructural polysaccharides found naturally as a storage carbohydrate. The natural occurrence of fructans producing plants used for human nutrition belong mainly to the composite (25,000 species) e.g., chicory, dahlia, Jerusalem artichoke and to the Liliacea (3500 species) e.g., onion and garlic [1]. Fructans in the plant roots, reserve carbohydrate stored in underground over-wintering organs and acts as cryoprotectant [1].
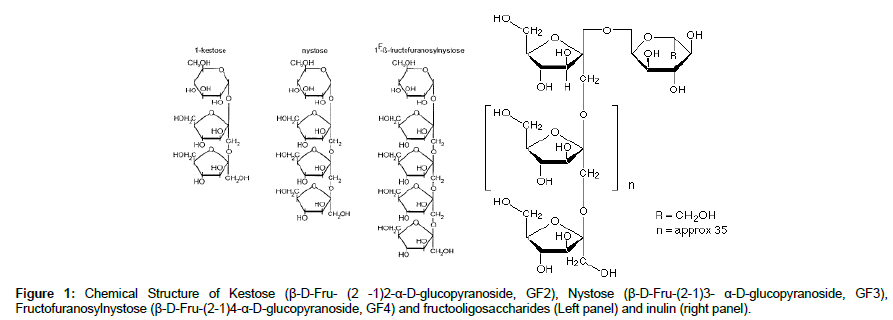
Fructans are linear plant oligo- and polysaccharides which consist of one terminal glucose as in a sucrose molecule that is elongated by a chain of fructosyl units connected through β-(2→1) fructosyl-fructose glycosydic bond [2]. The degree of polymerization and molecular weight depend on the source, the cold climatic conditions and the harvest time. The structure of fructo-oligosaccharides (FOS) and inulin (INU) have potentially beneficial nutritional effects, which meet the three classification criteria for being considered a prebiotic (Figure 1) [3]. These criteria are 1) resistance to gastric acidity, to hydrolysis by mammalian enzymes, and to gastrointestinal absorption; 2) selective fermentation of β (2-1) fructans by most Bifidobacterium species and also by some Lactobacillus species [4]. Growth of this bacterial population has been found to influence the health and nutrition of the host due to the supply of nutrients, conversion of metabolites, and interactions with host cells [5]. Short-chain fatty acids (SCFA) are the principal products of fermentation in the large intestine of man and other animals and they are the predominant anions in the colon, with acetate, propionate and butyrate occurring in greatest amounts [6]. The fermentation of onion was associated with significant increases in the production of short chain fatty acids and lowering the gut pH in rat trial that contribute to health and well-being. Dietary carbohydrates that show prebiotic ability include fructans- fructooligosaccharides (FOS) and inulin.
Well-known effects of inulin-type fructans on the gut microbiota are management of endothelial dysfunction, reducing serum cholesterol concentration and inducing hypolipidemia, enhance the immune system [7-12]. Consumption of dietary FOS enhances intestinal calcium absorption in experimental rats and enhances femoral bone volume and mineral concentration [13-16]. This increased absorption takes place mainly in the large intestine [17]. This Allium vegetable has been proven to have beneficial health effects including antioxidant and antimicrobial activities [18].
Objectives
The objective of the present study is to develop simple technology for the production of onion preparation rich in FOS and INU with prebiotic activities and to assess the prebiotic activities in rat feeding trial.
Methods and Materials
Onion (Allium cepa L.; Family Liliaceae) bulbs with golden outer coats were purchased from the retail market as one batch weighing 5 kg. The edible parts of the onion bulbs were chopped electrically and a portion was saved for the determination of moisture [19]. The dry onion was ground electrically and saved in air tight containers for summative chemical analysis according to AOAC methods [19]. The moisture content was used to express the onion composition as fresh and on dry matter basis.
Extraction of onion FOS and inulin
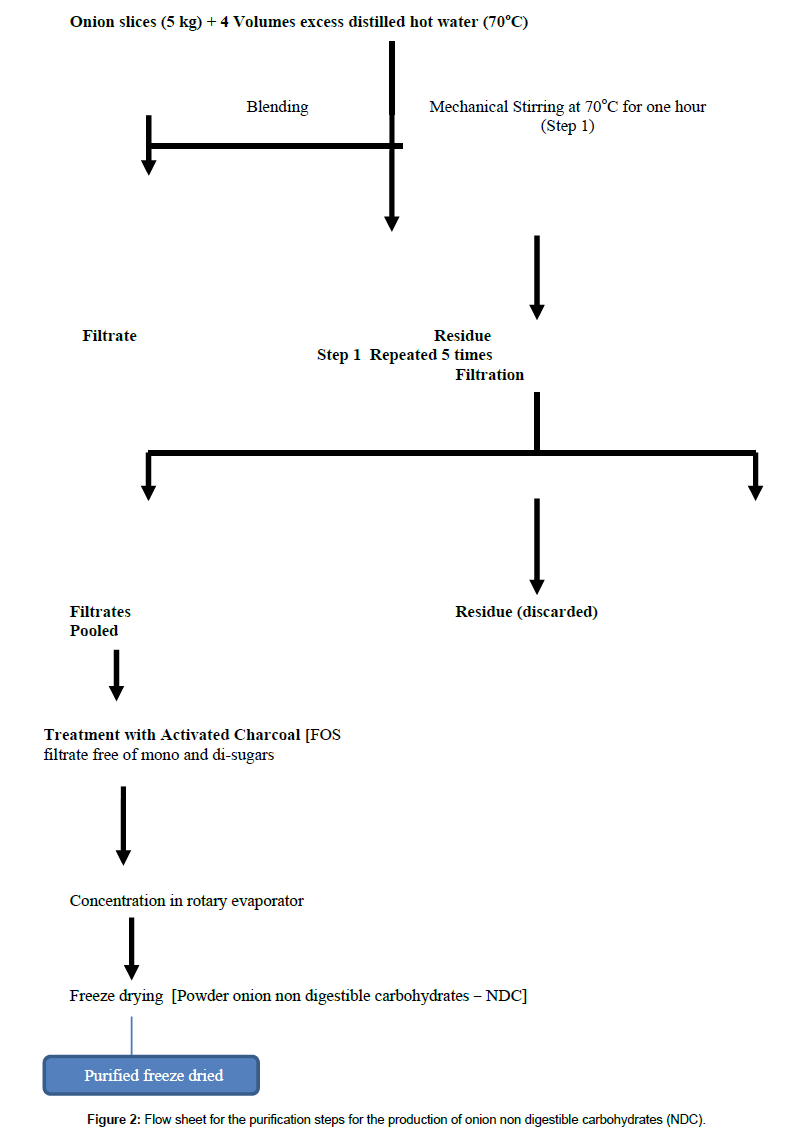
The peeled onion bulbs were cut down and homogenized in a Waring Blender with 5 liters of hot water (70°C) for 5 min. The homogenate was placed in a water bath adjusted to 75°C for one hour with continuous mechanical stirring at 500 rpm followed by filtration under vacuum. The cake (1.5 kg) was resuspended in 2.5 liters of hot water and the blending step, heating in water bath and filtering under vacuum was repeated 4 times until the final weight of the wet cake was reduced to 826 g. Aliquots of all extracts and of the cake were saved frozen for subsequent biochemical analysis. The six extracts were pooled to give a final volume of 14 L, which were passed through a column packed with activated coal to adsorb mono sugars and disaccharides. The volume of the clean-up elute was reduced down to 640 ml using rotary evaporator under vacuum and a temperature of 40°C. The viscous syrup was lyophilized on a freeze drier (Heto – Denmark) and the details of the process are illustrated in the flow sheet (Figure 2).
Pilot scale
The preparation of onion non digestible carbohydrates was done on a pilot scale at the pilot plant-Division of Engineering Research- National research center through the courtesy of Professor Giselle El- Diwany, Head of the Department. One ton of fresh onion was used as the starting material and the outer coat was removed mechanically. The bulb was cut into pieces and placed in a thermostated (70°C) stainless steel reactor 300 Liter capacity. Hot water was added in 5 fold excess (250 liters). Mechanical stirring continued for one hour followed by centrifugation. The supernatant was separated by aspiration and saved in the cold. The cake (7 kg) was extracted with 5 liters of hot water under the same conditions. The supernatants were combined (130 Liters) and were passed upstream through a column packed with activated coal to adsorb mono sugars and disaccharides. The Elute (127 liters) was concentrated using Liquid liquid extraction system down to 50 liters. This volume was concentrated further in an open vessel to a final volume of 8 liters.
The HPLC equipment (Knauer, Germany) equipped with a C18 stainless steel column (25 cm × 4 mm, id) packed with NH2P (amino propyl polymer) with particle size 5 μm. The injection volume was 20 μl and the mobile phase consisted of acetonitrile: water at a ratio of 70:30 (v/v) at a flow rate of 0.8 ml/min and at temperature of 20°C using an HPLC pump (K-501 Knauer). The elutes were monitored using the refractometer (RI K-23-1 Knauer) and signals from the detector were recorded simultaneously by the data system Euro Chrom 2000. Chromatographic peaks of fructose, glucose, sucrose, FOS, kestose; nystose and fructofuranosylnystose were quantitated by measuring peak area and comparing them to a standard curve generated by plotting area counts against concentration of standards using Euro Chrom 2000 program. FOS (Wako pure, Japan) solution was dissolved in distilled water 20.45 mg/ml and was run three times under the same condition to create the calibration curve and to calculate the respective onion FOS compounds by the external standard curve procedure. The separation of onion inulin was done on the same HPLC instrument equipped with another column packed with Aminex HPX-87C and heated to 85°C using thermostated jacket. Injection volume was 0.5 ml and the elution was done with distilled hot water (85°C) as the mobile phase at a constant flow rate of 0.5 ml/ min. Otherwise all other conditions were identical to those described above for the separation of FOS. Pure Inulin (Novartis, Belgium) was dissolved in distilled water (20 mg/ml) and was run in parallel with the unknown samples and was eluted with retention time of 8.23 min. The concentration of onion inulin was determined by the external standard curve procedure.
Rat feeding trial
Male Sprague-Dawley rats with an initial body weight of 70 ± 10 g (Animal House Colony-NRC, Egypt) were housed individually in metabolic stainless steel cages in an environmentally controlled room at 22°C with a 12 h light, dark cycle and allowed free access to double distilled deionized water. This study was carried out in accordance with the center of health guide for the care and use of laboratory animals. The rats were maintained for one week on an adaptation diet consisting of 50% of the commercial diet and the other 50% a mixture of equal proportions of the four experimental diets. The rats were then divided randomly into four groups of equal numbers. The first group was maintained on the control diet (Table 1), the 2nd group to the basal diet the onion purified non digestible carbohydrates preparation (NDC) was incorporated at a level of 13% at the expense of starch. The 3rd group and 4th groups consumed diet containing commercial FOS and inulin at 10% and 10% levels, respectively. The composition of the vitamin mixture followed AIN-93 and the mineral mixture [19,20]. The rats consumed the diets for five weeks and on the last week, the stool and urine were collected by connecting the cages to the stainless steel metabolic funnel with glass wool at the bottom and placing plastic container below the funnel for urine collection. During the 5th week, food intake was recorded daily. Timed 24 h excretions of stool and urine were collected using standard methods. At the termination of this week, the animals were sacrificed with diethyl ether and the abdomen was opened, the caecum excised and washed with sterile physiologic saline, dried between two filter papers and its weight was recorded. The contents of the colon were emptied by gentle squeezing and weighed and then stored under CO2 in sealed bottles at -40°C for subsequent assay of the glycolytic enzymes.
| Ingredients | Basal | Onion NDC | FOS | Inulin |
|---|---|---|---|---|
| 0.4% Ca | 0.4% Ca | 0.4% Ca | 0.4% Ca | |
| Casein | 140 | 140 | 140 | 140 |
| Corn oil | 50 | 50 | 50 | 50 |
| Cellulose | 40 | 40 | 40 | 40 |
| Sucrose | 83.71 | 71.5 | 83.7 | 83.7 |
| Fructose | 13.15 | ـــــ | 13.15 | 13.15 |
| Glucose | 3.13 | ـــــ | 3.13 | 3.13 |
| Onion-NDC | ـــــ | 128.5 | ـــــ | ـــــ |
| FOS, Novartis | ـــــ | ـــــ | 100 | |
| Inulin, Novartis | ـــــ | ـــــ | 100 | |
| Vitamin Mixture | 10 | 10 | 10 | 10 |
| Mineral Mixture | 35 | 35 | 35 | 35 |
| Maize Starch | 625 | 525 | 525 | 525 |
| Total | 999.99 | 1000 | 999.98 | 999.98 |
Table 1: Composition of the diets for the rat feeding trial.
Bone Density, Size and Mechanical Properties Right femurs and tibiae were measured for length and width at the midshaft using an ABS digimatic solar caliper (Tri-State Instrument Service, Fort Wayne, TX). Bone strength was assessed using a 3-point bending test on a materials testing machine (Alliance RT/5, MTS Systems, Eden Prairie, MN). Bone calcium content after mechanical testing, right femurs and tibias were dissolved in concentrated nitric acid overnight, diluted with ultrapure water and analyzed for total calcium content using atomic absorption spectrometry.
Calcium balance and kinetics
To assess chronic effects of dietary onion (NDC), FOS and INULIN on calcium absorption and metabolism, the metabolic balance was performed during the last week of the feeding study, urine and feces were collected for 24 h. The following parameters were calculated: Apparent mineral absorption, %=[(Ca intake-fecal Ca excretion)/Ca intake] × 100
Apparent Ca balance, mg/d=Ca intake-[fecal Ca excretion+urinary Ca excretion]
Apparent Ca balance, % absorption=[apparent Ca balance in mg/d/(apparent Ca absorption × mineral intake)] × 100% Apparent mineral balance data are also presented as a percent of absorption.
Chemical analysis
Onion and onion extracts were analyzed for total hydrolysable carbohydrates by the anthrone [19]. Glucose content in onion extract was assayed enzymatically by glucozyme kits (Bio Analytics, U.S.A) according to instructions of the manufacturer. Sucrose was assayed enzymatically using invertase [21]. The liberated glucose was assayed enzymatically using glucozyme reagent.
Assay of Caecal glycolytic enzyme activities
Caecal tissue (0.25 g) was homogenized with 5.0 ml of ice cold 0.1 M sodium phosphate buffer containing 0.15 mol sodium chloride, pH7.4 and sonicated (Probe sonicator,Vibra Cell, Sonics) in the cold for 2 min. Caecal enzymes activities were measured by preparing 5 tubes each containing aliquots (0.2 ml) of the sonicated homogenate followed by incubation for 10 min in water bath at 37°C with 100 μl of one of the following substrate solutions (5.0 mm) p-nitrophenyl- α-D-glucopyrannoside, p-nitrophenyl-β-D-glucopyrannoside, p-nitrophenyl-α-D-galactopyrannoside, p-nitrophenyl-β-Dgalactopyrannoside, and p-nitro-α-D-glucuronide as the substrates of the enzymes– α-glucosidase, β–glucosidase, α–galactosidase, β– galactosidase and α-glucuronidase, respectively. The enzymatic assay was terminated after 10 min by adding 1.6 ml of sodium carbonate solution (10%). After centrifugation at 1000 rpm for 5 min, the absorbancy of the clear yellow supernatant was measured against blank at 400 nm using a Shimadzu spectrophotometer (UV-120-02). The caecal enzymatic activities were expressed as μM of p-nitrophenol liberated per minute per gram of caecal protein. Total soluble protein concentration in the sonicated caecal homogenates was determined after appropriate dilutions according to the method of Lowry et al. using bovine serum albumin as standard [22].
Analysis of calcium in the diet, feces, urine and bones [23]. Wet ashing by heating about 0.5 g of the finely ground diet, feces and bones in Teflon vials containing a mixture of 3.0 ml nitric acid (65%, ultrapure, Merck, Darmstadt, Germany) and 2.5 ml hydrogen peroxide. Wet ashing was done in a microwave oven using appropriate program according to instruction of the manufacturer (ETHOS 900, Milestone, Italy).The clear hydrolysate was diluted to final concentration of 3% HNO3 containing 0.8% lanthanum chloride. The AAS of calcium was measured using flame atomic absorption spectrometer (Perkin Elmer 300) at wavelengths of 422.7 nm and slit width of 0.5 mm with 10 mA for the lamp current by aspirating the clear digest through the flame using nitrous oxide acetylene gas. Urinary Ca was determined without pretreatment by aspirating the filtered urine sample directly through the flame. Standard calcium solution (titrisol) was purchased in concentration of 1000 ± 0.002 mg/L (Merck (Darmstadt, Germany) and the working Ca solutions were prepared after appropriate dilutions and were run in parallel and served to calculate the concentration of calcium by the external standard curve procedure.
Food intake and feces recorded during the last week of the experiment were used to calculate the apparent Ca absorption %)= (CaI–Fecal Ca)/CaI] × 100, whereby, Ca I=calcium intake.
Bone strength and density
The length and thickness of the right femur were measured by a Caliper [24]. The bone strength was measured using a digital Force Gauge (Nidec-shimpo-corporation, Japan). The bone density was measured by dual energy X-ray absorptiometry (DXA).
Statistical analysis
Values are presented as the arithmetic means and SEM and differences. The one way analysis of variance (ANOVA) was conducted using the general linear model procedure to assess the differences in the effect of the three dietary supplements (Onion NDC, FOS or inulin) and control on outcome measures. The models included the main effects of treatment. Pearson correlation was used in the correlation evaluations. All evaluations were performed with SPSS version 18.
Result
The total soluble carbohydrates in the fresh onion averaged 12.65%; representing 85.5% of the total solid (Table 2). The purification steps for extracting the onion non digestible carbohydrates (Table 3) showed that 89.8% of the onion soluble carbohydrate was extracted in the first extract. Six time extraction recovered over 97.13% of the total onion soluble carbohydrate as determined by the colorimetric anthrone method.
| Nutrient | Unit | Content |
|---|---|---|
| Moisture | % | 85.20 |
| Dry matter | % | 14.80 |
| Energy | Kcal | 43.47 |
| Energy | KJ | 181.71 |
| Protein | % | 1.13 |
| Crude ether-extract | % | 0.190 |
| Ash | % | 0.617 |
| Soluble carbohydrates* | % | 12.65 |
| Calcium | % | 0.179 |
Table 2: Composition of fresh onion.
| Purification step | Water for extraction, L | Total soluble carbohydrates, g | Soluble carbohydrates, % | Soluble carbohydrates ** |
|---|---|---|---|---|
| Fresh onion bulbs*, 5 kg | 632.4 | 12.65 | 100 | |
| Extract 1st | 7 | 567.7 | 8.11 | 89.77 |
| Extract 2nd | 3 | 39.9 | 1.33 | 6.31 |
| Extract 3rd | 1.1 | 3.08 | 0.28 | 0.49 |
| Extract 4th | 0.98 | 1.96 | 0.2 | 0.31 |
| Extract 5th | 0.98 | 1.47 | 0.15 | 0.23 |
| Extract 6th | 0.900 | 1.26 | 0.14 | 0.20 |
| Total extracts | 13.96 | 615.37 | 97.31 | |
Table 3: Purification steps and recovery of onion soluble carbohydrates in the different extracts.
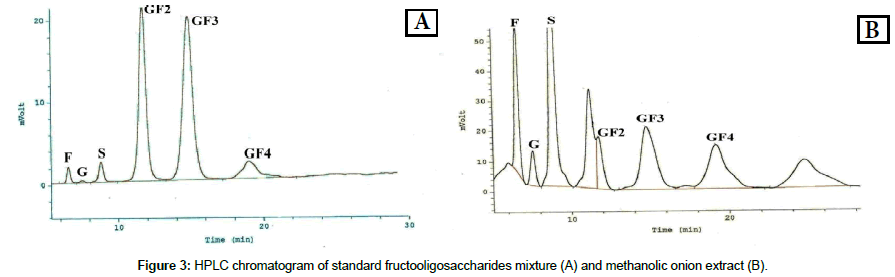
Figure 3 is a typical HPLC chromatogram for onion FOS illustrating the peaks of kestose (GF2), nystose (GF3) and fructofuranosylnystose (GF4) with the respective retention times of 11.72; 14.84 and 19.0 min. The nystose represents the largest fraction, followed by kestose. Fructose, glucose and sucrose peaks appeared as impurities and denotes that they escaped adsorption on the activated charcoal.
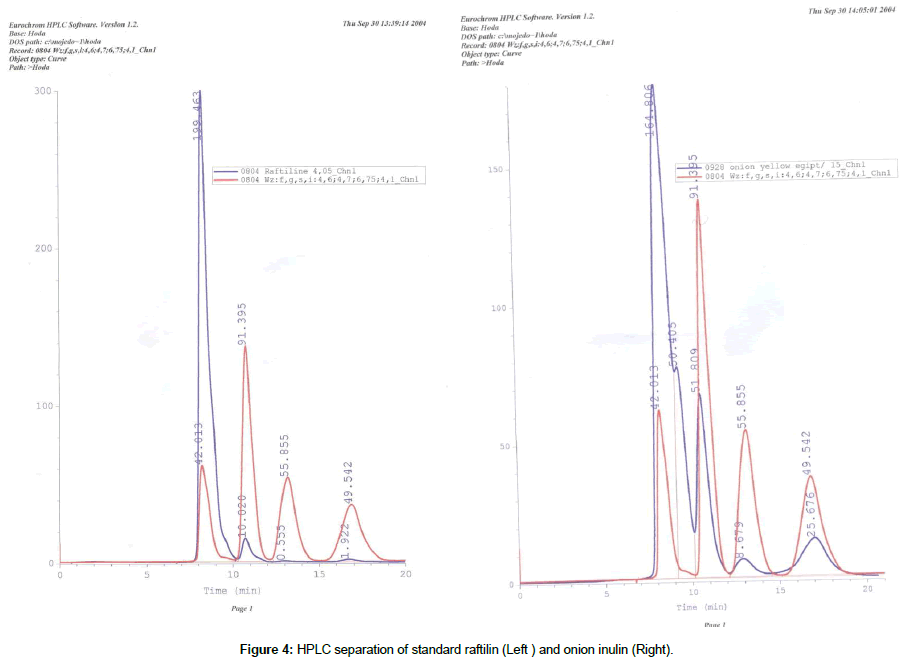
The HPLC separation of standard inulin (Raftilin) resulted in 5 broad peaks (Figure 4) with retention times of 8.23, 9.3, 10.6, 12.9 and 17.06 min and the peaks were almost overlapping the retention time of the onion inulin fractions. The contents of the total FOS fractions and inulin averaged 23 and 15.5%, respectively in the freeze dried onion (NDC) preparation with a ratio of 1.516 : 1 (w/w) (Table 4).
| Ingredients | Onion, Giza | Onion, Tantawi | Mean |
|---|---|---|---|
| Onion purified non digestible carbohydrate, g/100 g dry matter | |||
| Kestose | 4.95 | 4.64 | 4.8 ± 0.22 |
| Nystose | 11.57 | 8.26 | 9.9 ± 2.34 |
| Fructosyl-nystose | 10.26 | 6.25 | 8.26 ± 2.83 |
| Total FOS | 26.77 | 19.15 | 22.96 ± 5.39 |
| Inulin | 15.49 | ND | 15.49 |
Table 4: Content of FOS and Inulin in the purified onion non-digestible carbohydrate.
Body and Cecal wall weight and microbial enzyme activities. Total food intake and gain in body weight were significantly lower in rats consuming onion (NDC) compared to all other groups (Table 5). The 5 week feeding clearly demonstrates that the caeca from rats fed the onion NDC, FOS and INULIN were significantly larger than those of the control group and the same applies when the caecum weight was expressed as % body weight. The stool bulk and hydration of stool were also significantly higher among the groups consuming onion (NDC), FOS or INU than those of the control group. The mean pH values of the content of the cecca from rats fed the onion NDC, FOS and INULIN were significantly lower than those of the control group. A comparison of the activities of selected caecal microbial enzymes demonstrated significant increases in the activities of α-, β-glucosidase; α-, β-galactosidase in μM per g of caecal protein than those of the control group. The activity of the caecal microbial β-glucuronidase was significantly reduced than those of the control group (P<0.05).
| Parameters | Control | Onion (NDC) | FOS | Inulin |
|---|---|---|---|---|
| Initial body weight, g | 81.7 ± 3.6 | 80.6 ± 3.5 | 81.7 ± 2.84 | 79.7 ± 4.11 |
| Final body weight, g | 200.8 ± 13.0b,c | 150.6 ± 5.0a | 186.1 ± 9.3b | 187.2 ± 6.7b |
| Gain in body weight, g/28 d | 119.1 ± 9.9b | 70.0 ± 6.07a | 104.3 ± 9.4,b | 107.5 ± 3.8b |
| Food intake /gain in body weight, g/g | 0.23 ± 0.01b | 0.17 ± 0.01a | 0.22 ± 0.01a,b | 0.23 ± 0.01b |
| Final caecum weight, g | 2.06 ± 0.16a | 3.54 ± 0.26b | 4.24 ± 0.43b | 4.57 ± 0.29b |
| Caecum, % body weight | 1.04 ± 0.08a | 2.38 ± 0.24b | 2.28 ± 0.21b | 2.44 ± 0.14b |
| Caecum content, g | 1.37 ± 0.15a | 2.57 ± 0.21b | 3.02 ± 0.39b | 3.08 ± 0.31b |
| Caecal pH | 6.70 ± 0.05b | 6.08 ± 0.05a | 5.99 ± 0.17a | 6.01 ± 0.01a |
| α- glucosidase, µmol/g prot | 12.54 ± 0.53a | 15.80 ± 0.45b | 16.34 ± 0.55b | 15.51 ± 1.11b |
| β- glucosidase, µmol /g prot | 6.10 ± 0.30a | 7.46 ± 0.51b | 8.23 ± 0.19c | 7.53 ± 0.43b |
| α- galactosidase, µmol /g prot | 7.23 ± 0.44a | 11.12 ± 0.58b | 13.19 ± 0.84b | 12.46 ± 1.05b |
| β- galactosidase, µmol /g prot | 10.28 ± 0.74a | 12.65 ± 0.66b | 14.99 ± 1.49b | 13.41 ± 1.39b |
| β- glucoronidase, µmol/g prot | 12.51 ± 1.55b | 8.65 ± 1.15a | 9.69 ± 0.90a | 10.80 ± 1.31a,b |
Table 5: Mean rat colon weights and gut microbial enzyme activities following consumption of prebiotic containing diets.
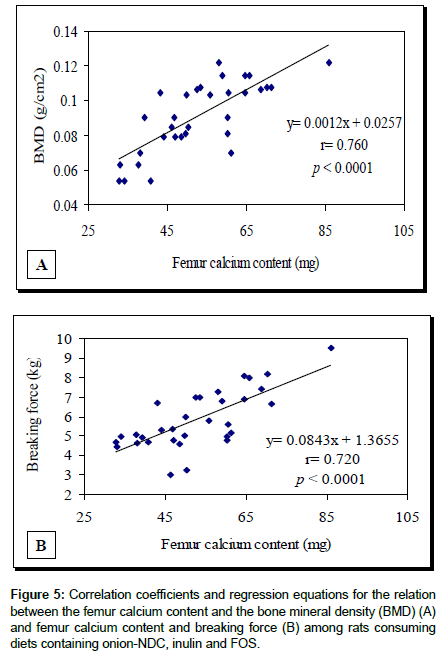
The results of the calcium metabolic study clearly demonstrate that the diets containing onion (NDC), FOS or INULIN significantly improved almost all parameters of calcium metabolism (Table 6). The fecal excretion of calcium was reduced significantly than those of the control group (P<0.05). Accordingly, the % calcium absorption and calcium balance (mg/ day/) and % calcium retention were significantly higher among the groups consuming onion (NDC), FOS or INU than those of the control group. Five of the 6 studied parameters of bone mass responded positively to the diets containing onion (NDC), FOS or INULIN and increased significantly in comparison with the respective control values (P<0.05). Very high significant correlation coefficients of 0.76 and 0.72 were found (P<0.001) between femur calcium and body mineral density (Figure 5a) and between femur calcium and breaking force, respectively (Figure 5b).
| Parameter | Diets | |||
|---|---|---|---|---|
| Control | Onion (NDC) | FOS | Inilin | |
| Food intake, g/ d | 14.6 ± 0.8 | 12.1 ± 0.7 | 13.4 ± 0.5 | 13.6 ± 0.7 |
| Ca intake, mg/d | 66.18 ± 3.5 | 57.04 ± 2.30 | 66.44 ± 2.04 | 58.93 ± 3.46 |
| Fecal Ca, mg/d | 33.16 ± 4.2b | 15.51 ± 1.35a | 14.32 ± 0.65a | 13.34 ± 1.29a |
| Urinary Ca, mg/d | 0.18 ± 0.03 | 0.20 ± 0.03 | 0.18 ± 0.02 | 0.18 ± 0.01 |
| Ca balance, mg/d | 32.84 ± 3.5a | 41.33 ± 1.1a, b | 51.94 ± 1.62c | 45.41 ± 2.4b,c |
| Apparent Ca retention % | 49.62 ± 4.8a | 72.46 ± 1.42b | 78.18 ± 0.69c | 77.06 ± 1.1b,c |
| Femur length, mm | 27.08 ± 0.48b | 25.50 ± 0.18a | 26.79 ± 0.14b | 26.58 ± 0.27b |
| Femur thickness, mm | 2.56 ± 0.07 | 2.62 ± 0.04 | 2.76 ± 0.05 | 2.70 ± 0.07 |
| Femur Ca mg/100 g | 47.73 ± 3.80a | 54.75 ± 3.8a, b | 66.64 ± 5.05b | 63.24 ± 4.23b |
| Tibia Ca % mg/100 g | 43.70 ± 3.1a, b | 61.30 ± 3.25b,c | 77.45 ± 2.81c | 69.85 ± 2.90c |
| Femur break strength (kg/cm2) | 4.90 ± 0.14a | 6.186 ± 0.26b | 7.94 ± 0.47c | 7.23 ± 0.27c |
| Bone mineral density BMD (g/cm2) | 0.08 ± 0.005a | 0.11 ± 0.001b | 0.12 ± 0.004b | 0.11 ± 0.001b |
One way analysis of variance (ANOVA) test.
Table 6: Mean calcium intake and apparent calcium balance and retention among rat groups following consumption of prebiotic containing diets.
Discussion
The use of FOS in the human diet has increased since the initial commercial production of a specific oligofructan (Neosugar) in Japan in 1983. The reports given have linked biochemical-nutritional health changes in human resulting from eating Neosugar [25]. A 2007 report on the world prebiotic market states that there are over 400 prebiotic food products and more than 20 companies producing oligosaccharides used as prebiotics and the European prebiotics market reached €179.7 million by 2010 [26]. The majority of studies have so far focused on inulin and FOS, since both compounds have now a long history of safe use .
Egypt with estimated annual production of 1,903,000 tonne belongs to the World’s top onion producing countries which amounts globally to 84,758,191 tons.
Onion is the second most eaten vegetable in Egypt right after tomato. The advanced analytical techniques help in product specification based on scientific evaluation of the functional and health properties of prebiotics. Variations in onion FOS and inulin composition exist in the reported literature and is attributed to different climatic conditions in the different geographical regions, where onion is grown [27-29]. According to one study onion fructan makes up 25- 40% of the non-digestible carbohydrates, while the% distribution of FOS GF2: GF3 to GF4: GF5-8 were 61:25:10:3, respectively [28]. In the present study, inulin made up 15.5 % of total onion (NDC) and the % respective distribution of FOS GF2: GF3 to GF4 were 21:43:36. Analysis of onion tissue fructooligosaccharides by advanced analytical tools led to an ideal separation and identification of the structural composition of nine up to 12 different fructooligosaccharides of onion bulb [29,30]. The technique of MALDI-TOF-MS is a powerful tool for the characterization of carbohydrates and to determine chain length distribution of food FOS and inulin both qualitatively and quantitatively [30,31].
The large intestine or the colon is by far the most colonized region of the gastrointestinal tract, with about 1012 bacteria per gram of gut content [32]. FOS with short chain length, unbranched nature and high solubility in water such as onion (NDC), FOS and INULIN exerted their action by diluting the caecal contents and also causing caecal expansion and constituting a carbon source for microbial flora of bowel water [33,34]. The significant enlargement in the caecca weights following consumption of diets containing onion NDC, FOD or INULIN reflects colonization with bifidobacteria in response to the bifidogenic effects of the supplements. Similar results had been reported by other investigators following the feeding of onion inulin [7,33].
Through the fermentation process, colonic bacteria, most of which are anaerobes, produce a wide variety of compounds that may affect gut as well as systemic physiology. Fermentation of nondigestible carbohydrates reaching the large bowel produces shortchain carboxylic acids mainly acetate, propionate and butyrate and lactate (SCFA), which are sources of energy for the tissue [35]. The energy supply allows the host to salvage part of the energy for regulating both cell division and cellular metabolism [36]. Nondigestible carbohydrates resisted endogenous digestion and reached the colon intact where they are fermented. The acidic caecal pH resulting from ingestion of the fermentable carbohydrate diets is caused by the greater level of total SCFA production [37].
An important property of short-chain fructooligosaccharides is the stimulation of bifidobacterial growth specifically while suppressing the growth of potentially harmful species such as, for example, Clostridium perfringens in the colon, a decrease in the production of nitrogenous end products in urine and stools. [38]. FOS consumption reduces colon turnout-development by enhancing both colon butyrate concentrations and local immune system [16,39]. The incorporation of non-digestible carbohydrates in rat diets increased significantly the activities of glycosidase enzyme and decreased significantly the activities of β-glucuronidase. In the present study, caecal glucuronidase activity was reduced significantly among the rat group consuming the onion NDC, FOS and INULIN compared with the respective control (P<0.05). β-Glucuronidase is an enzyme responsible for the hydrolysis of glucuronides in the lumen of the gut and this reaction generates toxic and carcinogenic substances which are detoxified by glucuronide formation in the liver and then enter the bowel via bile. In this way, toxic aglycones can be regenerated in the bowel by bacterial β-glucuronidase and in humans, the fecal β-glucuronidase activity was shown to be higher in colorectal cancer patients in comparison with healthy controls suggesting a role of this enzyme in carcinogenesis [40,41].
This reduction in caecal pH leads to greater solubilization of Ca so that the biologically available concentration of these minerals is increased [42].
The higher absorption of calcium from the colon was attributed to the lowering in caecal pH value following the consumption of the onion NDC, FOS and INULIN compared with the respective pH value of the control (P<0.05).
Compared to the prebiotic free control diet, dietary onion (NDC) decreased significantly, the mean caecal pH values from 6.7 in the control down to pH values of 6.0–6.08 in the caecum of the 3 other groups. mean activities of the four colonic enzymes α-glucosidase; β-glucosidase; α-galactosidase and β-galactosidase increased significantly in the rat groups consuming the onion NDC, FOS and INULIN containing diets compared with the respective activities of the control (P<0.05). On the contrary, the mean colonic β-glucuronidase activity was significantly (lower among rats fed the prebiotic containing diets compared with the respective activity among the control (P<0.05). Similar findings were reported earlier.
The administration of onion NDC at 13% of the diet led to 41.2% lower growth rate than those on control diets, which is in agreement with previous findings [43]. Other investigators could not find significant differences neither in the body weight gain between the control group and those on inulin, resistant starch or on diets containing a mixture of kestose, nystose at 10% of the diet for six weeks [44,45].
The interest in Raftilose from mainstream nutritionallyorientated companies is mainly for its calcium absorption properties, as well as its use as an easy-to-incorporate fiber that is undetectable in the finished product. Those taking the supplement had 15 percent greater calcium retention and accretion in their bones. Calcium consumption is particularly important in early adolescence in order to achieve an optimal peak bone mass. A detailed study showed that the soluble fraction of cecal Ca was 3-4 times higher in the groups receiving inulin than those without inulin [43].
A balance study showed significant reduced fecal calcium excretion associated with significant high calcium absorption (%) after feeding experimental rats diets containing either 10% kestose, nestose or FOS for two weeks [45].
Commercial inulin (10%) in the diet increased colonic absorption of calcium in rats [44]. A study reported that 10% dietary inulin– FOS mixture increased the large intestinal calcium absorption by enhancing pools of soluble and ionized calcium, an increase in the absorptive surface predominantly in caecum, the increased concentrations of SCFA, and by direct interaction with the intestinal tissue [15]. A study showed that only diets containing 5% inulin and not FOS potentially improved calcium absorption, BMD, BMC of excised femurs and BMD of the cortical bone as well as the polar stress/strain index of femurs and attributed these positive effects to the higher capacity of these fructans to reduce bone resorption [46].
These results clearly indicate that fermentation of inulin in the colon to short chain fatty acids was the main reason for the increase in mineral absorption.
A recent investigation demonstrated that in growing rats receiving either a control diet or a diet enriched with 5% oligofructose (OLF) or 5% inulin for 3-month period, showed that the inulin group was superior with regard to the increase in whole-body bone mineral content (BMC) as measured by DXA. Furthermore, dietary fructans improved calcium absorption and BMD in rats and an increase in the BMC of excised femurs and BMD of the cortical bone in both appendicular and peripheral sites (P<0.01) as well as the polar stress/strain index of femurs (P<0.01was detected only in the inulin group and not in the OLF group [46]. Nzeussen based on the above-mentioned results, the authors suggested that the greatest effect of inulin is related to the higher capacity of this fructan to reduce bone resorption.
In the present study, apart of the effect of inulin containing diets on calcium retention in rats, there was an increase in total femoral Ca in the inulin group, which translated into higher femoral BMD and breaking strength.
Similar findings were reported in the literature for the positive effect of a mixture of inulin and FOS (4.5% in the diet) on calcium absorption, retention and total femoral calcium and density in ovarictomized rats. Bone is constantly being turned over by two cell types, i.e. the osteoblasts and the osteoclasts. The osteoblasts, which are derived from mesenchymal stem cells, are able to form organic bone matrix onto which mineral is deposited. Both can be degraded by the osteoclasts, which are derived from hemopoietic progenitors. In healthy bones, this continuous process of bone formation and bone resorption is in equilibrium and thus ensuring maintenance of bone mass. However, this equilibrium can seriously be disrupted, either through increased or decreased bone formation or bone resorption, which leads to pathological changes in bone mass. These results were obtained with animal models and they require further basic studies to be extrapolated to humans.
Conclusion
Bifidogenic effects are not sufficient without demonstrated physiological health benefits and the assessment of metabolic functions of the gut flora, such as bacterial enzyme activities and bacterial metabolites, is more relevant to the functions of the gut microbiota on human health than the classical bacteriological counts. Determining events that take place within compartments of the intestine are often difficult. Until such times as specific site sampling or more sophisticated methods can reliably link microbiota modulation with health benefits, faecal analysis will be deemed suitable. Inulin and oligofructose are not measured by classic methods of dietary fiber analysis and consequently are often not mentioned in food tables. After establishing its chemistry and health benefits, it is evident that onion FOS fit well within the current concept of the class of dietary material and could be labeled as “functional foods’ since their vast health benefits are continuously appreciated. Due to their specific properties, onion NDC affects several functions and contributes to reduce the risk of many diseases. Thus, they may contribute in a significant way to a well-being by their specific effects on several physiological functions. According to local legislation, products having history of safe use in the target host, such as GRAS or its equivalents, do not require further animal and human toxicological studies. The significant contribution of FOS and INULIN to the dietary fiber fraction (recommended at 25 g/d/per capita) is not taken into account in any nutritional recommendations. In view of this, inulin and oligo-fructose deserve more attention, both in food composition tables and in diet or nutrition studies.
References
- Hendry AF, Wallace RK (1993) The origin, distribution and evolutionary significance of fructans. CRC, United Kingdom.
- Kelly G (2008) Inulin-type prebiotics--a review: Part 1. Altern Med Rev 13: 315-329.
- Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic icrobiota: Introducing the concept of prebiotics. J Nutr 125: 1401-1414.
- Kaplan H, Hutkins RW (2000) Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Appl Environ Microbiol 66: 2682-2684.
- Flint HJ, Duncan SH, Scott KP, Louis P (2007) Interactions and competition within the microbial community of the human colon: links between diet and health. Environ Microbiol 9: 1101-1111.
- Cummings JH, Macfarlane GT (1991) The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol 70: 443-459.
- Pascoal GB, Filisetti TMCC, Alvares EP, Lajolo FM, Menezes EW, et al. (2013) Impact of onion (Allium cepaL) fructans fermentation on the cecum of rats and the use of in vitro biomarkers to assess in vivo effects. Bioactive Carbohydrates Dietary Fibre 1: 89-97.
- Catry E, Bindels LB, Tailleux A, Lestavel S, Neyrinck AM, et al., (2017) Targeting the gut microbiota with inulin-type fructans: preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut 67: 271-283.
- Costa GT, Castro de, Abreu G, Guimarães ABB, Vasconcelos PRL, et al. (2015) Fructo-oligosaccharide effects on serum cholesterol levels. An overview. Acta Cirúrgica Brasileira 30: 367-378.
- Delzenne NM, Kok NN (1999) Biochemical basis of oligofructose- induced hypolipidemia in animal models. J Nut 129: 1467S-1470S.
- Slavin J (2013) Fiber and prebiotics: Mechanisms and health benefits. Nutrients 5: 1417-1435.
- Seifert S, Watzl B (2007) Inulin and oligofructose: Review of experimental data on immune modulation. JNutr 137: 2563-2567.
- Scholz-Ahrens KE, Schaafsma G, Ellen Heuvel GHM, Schrezenmeir J (2001) Effects of prebiotics on mineral metabolism. Am J Clin Nutr 73: 459-464.
- Zafar TA, Weaver CM, Jones K (2004) Inulin effects on bioavailability of soy isoflavones and their calcium absorption enhancing ability. J Agric Food Chem 52: 2827-2831.
- Raschka L, Daniel H (2005) Mechanisms underlying the effects of inulin – type fructans on calcium absorption in the large intestine of the rat. Bone 37: 728-737.
- Takahara S, Morohashi T, Sano T, Ohta A, Yameda S, et al. (2000) Fructooligosaccharide consumption enhances femoral bone volume and mineral concentration in rats. J Nutr 130: 1792-1795.
- Mc Cabe L, Britton RA, Parameswaran N (2015) Prebiotic and probiotic regulation of bone health: Role of the intestine and its microbiome. Curr Osteoporos Rep 13: 363-371.
- Fouad M, Moustafa A, Hussein L, Romeila R, Gouda M, et al. (2015) In vitro antioxidant and antimicrobial activities of selected fruits and vegetable juices and fermented dairy products commonly consumed in Egypt. RJPBCS 6: 541-550.
- Association of Official Analytical Chemists (1990) In Official Methods of Analysis (15th edn), USA.
- Reeves PG (1997) Components of the AIN-93 Diets as Improvements in the AIN-76A Diet. J Nutr 127: 838S-841S.
- Cerning-Beroard J (1975) The use of invertase for determination of sucrose, Application to cereals, cereal products, and other plant materials. Cereal Chem 52: 431-438.
- Lowry OH, Rosebrough NJ, Farr A L, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265
- Gough DS, Hannaford P, Lowe RM (1989) Studies of sputtering atomizers for atomic absorption spectroscopy. Anal Chem 61: 1652-1655.
- Zafar TA, Weav CM, Zhao Y, Martin BR, Wastney ME, et al. (2004) Nondigestible oligosaccharides increase calcium absorption and suppress bone resorption in ovariectomized rats. J Nutr 134: 399-402.
- Buddington KK, Donahoo JB, Buddington RK (2002) Dietary oligofructose and inulin protect mice from enteric and systemic pathogens and tumor inducers. J Nutr 132: 472-477.
- Bornet FRJ, Brouns F, Tashiro Y, Duvillier V (2002) Nutritional aspects of short-chain fructooligosaccharides: natural occurrence, chemistry, physiology and health implications. Digest Liver Dis 34: S111-S120.
- Jaime L, Martınez F, Martın-Cabrejas MA, Molla E, Lopez-Andreu FJ, et al. (2000) Study of total fructan and fructooligosaccharide content in different onion tissues. J Sci Food Agric 81: 177-182.
- Benkblia, Shiomi N (2006) Hydrolysis kinetic parameters of DP 6, 7, 8, and 9−12 fructooligosaccharides (FOS) of onion bulb tissues. Effect of temperature and Storage Time Current Nutr Food Sc 2: 181-191.
- Stahl B, Linos A, Karas M, Hillenkamp F, Steup M, et al. (1997) Analysis of fructans from higher plants by matrix-assisted laser desorption/ionization mass spectrometry. Anal Biochem. 246: 195-204.
- Borromei C, Careri M, Cavazza A, Corradini C, Elviri L, et al. (2009) Evaluation of fructooligosaccharides and inulins as potentially health benefiting food ingredients by HPAEC-PED and MALDI-TOFMS. Intern J Anal Chem 639: 1-9.
- Bäckhed F, Fraser CM, Ringel Y, Sanders ME, Sartor RB, et al. (2012) Defining a healthy human gut microbiome: Current concepts, future directions, and clinical applications. Cell Host Microbe 12: 611-622.
- Nyman M (2002) Fermentation and bulking capacity of indigestible carbohydrates: the case of inulin and oligofructose. Br J Nutr 87: S163-SA 168
- Ito H, Takemura N, Sonoyama K, Kawagishi H, Topping DL, et al. (2011) Degree of polymerization of inulin-type fructans differentially affects number of lactic acid bacteria, intestinal immune. J Agric Food Chem 59: 5771-5778.
- Macfarlane GT, Cummings JH (1991) The colonie flora, fermentation and large bowel digestive function. Raven, New York.
- Biedrzycka E, Bielecka M (2004) Prebiotic effectiveness of fructans of different degrees of polymerization. Trends Food Sc Technol 15: 170-175.
- Fanning S, Halla LJ, Croninc M, Zomera,A, MacSharrya J, et al. (2012) Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. PNAS 109: 2108-2113
- Fukuda S, Toh H, Taylor TD, Ohno H, Hattori M, et al. (2012) Acetate producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes 3: 449-454.
- Goldin BR, Gorbach SL (1976) The relationship between diet and rat fecal bacterial enzymes implicated in colon cancer. J Natl Cancer Inst 57: 371-375.
- Kim DH, Jin YH (2001) Intestinal bacterial beta-glucuronidase activity of patients with colon cancer. Archive Pharmacol Res 24: 564-567.
- Cashman K (2003) Prebiotics and calcium bioavailability. Microbiol 4: 21-32.
- Coudray C, Tressol JC, Gueux E, Rayssiguier Y (2003) Effects of inulin-type fructans of different chain length and type of branching on intestinal absorption and balance of calcium and magnesium in rats. Eur J Nutr 42: 91-98.
- Younes H, Coudray C, Bellanger J, Demigné C, Rayssiguier Y, et al. (2001) Effects of two fermentable carbohydrates (inulin and resistant starch) and their combination on calcium and magnesium balance in rats. Br J Nutr 86: 479-485
- Ohta A, Ohtsuki M, Baba S, Adachi T, Sakata T, et al. (1995) Calcium and magnesium absorption from the colon and rectum are increased in rats fed fructooligosaccharides. J Nutr 125: 2417-2424.
- .
- Wolf BW, Firkins JL, Zhan X (1998) Varying dietary concentrations of fructooligosaccharides affect apparent absorption and balance of minerals in growing rats. Nutrition Res 18: 1791-1806.
- Mundy GR (1999) Bone remodeling. (4th edn), Lippincott Williams Wilkins, Newyork.