Review Article, Biomater Med Appl Vol: 1 Issue: 2
Mesenchymal Stem Cells Associated with Bioceramics for Bone Tissue Regeneration
Daniel Navarro da Rocha1, Rubens Lincoln Santana Blazutti Marçal1, Rafael Maza Barbosa2, José Ricardo Muniz Ferreira1,2 and Marcelo Henrique Prado da Silva1*
1Instituto Militar de Engenharia, IME, Pça General Tibúrcio 80, P. Vermelha, Urca, Rio de Janeiro, RJ, Brazil, CEP 22290-270
2R-CRIO Criogenia S.A., Rua Cumaru 204, Alphaville, Campinas, SP, Brazil, CEP: 13098-324
*Corresponding Author : MH Prado da Silva
Military Institute of Engineering-IME, Materials Science Post Grade Programme, Brazil
E-mail: marceloprado@ime.eb.br
Received: October 14, 2017 Accepted: December 01, 2017 Published: December 08, 2017
Citation: Rocha DN, Marçal RLSB, Barbosa RM, Ferreira JRM, Silva MHP (2017) Mesenchymal Stem Cells Associated with Bioceramics for Bone Tissue Regeneration. Biomater Med Appl 1:2.
Abstract
Bioactive bioceramics are a family of osteoconductive materials that include bioactive glass and calcium phosphates such as hydroxyapatite (HA) and tricalcium phosphate (β-TCP or α-TCP). These materials exhibit excellent bone binding properties and are usually designed to be used as bone fillers or as bioactive coatings on metal implants. Their in vivo resorption ability may be modulated by controlling synthesis and processing parameters. Bioceramics can be associated to stem cells in cell-based therapies, using transplanted cells to guide the spatially complex process of tissue formation, thus optimizing osteoinduction, osteoconduction, and osteogenesis. Although tissue engineering strategies using stem cells aim to ameliorate the prognosis of the grafted materials, there are several questions still to be addressed, such as which are the best materials and which are the best adult stem cells to be associated with these scaffolds in order to improve and hasten the replacement of bone fillers by newly formed bone tissue in an ideal way. Almost all structures such as bone, cranial and facial sutures, cartilage, ligaments, and teeth, are derived from mesenchymal cells. Mesenchymal stem cells (MSCs) have been isolated from most of the postnatal tissues, including several craniofacial tissues. They have a well-characterized potential to differentiate into all cellular lineages that constitute mesenchymal and connective tissues. Dental pulp tissue is an interesting source of MSCs to be used in tissue bioengineering studies due to their multi-differentiation potential, noninvasive and efficient process of isolation, immunosuppressive activity and similarity to bone cells. The aim of this work is to discuss the potential of mesenchymal stem cells to be used for guided osteogenesis and show the efficiency of its association with fabricated scaffolds to aid bone regeneration processes.
Keywords: Mesenchymal stem cells; Dental pulp stem cell; Biomaterials; Bone regeneration
Introduction
Since the 1950’s, the advances of the clinical infrastructure and biomedical applications for health improvements have demonstrated an increase in life expectancy of world’s population. However, the increase in longevity was accompanied by a demographic transition, indicating an intrinsic concept of epidemiological transition [1]. This transition process is described as a progressive evolution of high mortality profile from infectious diseases to chronic-degenerative diseases. The current relevance of degenerative diseases and mortality patterns worldwide is a major concern for the international scientific community. Nowadays, adult stem cells have been presented as a key approach for the prevention, control and treatment of several kinds of degenerative diseases [2]. Adult stem cells brought a new perspective on different stem cell-based therapies, avoiding the ethical dilemma of the possible disposal of an embryo that occurs when embryonic mesenchymal stem cells are used [3]. Although adult stem cells show lower versatility to differentiate when compared to embryonic mesenchymal cells, an increase in their utilization as platforms to study disease and drug discovery or direct therapeutic use in association with different types of frameworks/delivery systems to regenerate damage tissues is verified [3,4].
In the recent decades, stem cell-based therapy has been widely used for the treatment of several disorders of the blood and immune system through adult hematopoietic stem cell transplants. A hematopoietic stem cell is a cell present in the blood able to renew itself and to differentiate into different blood cells: such as red blood cells, white blood cells and platelets. However, adult mesenchymal stem cells have been identified in specific areas of several tissues in order to assist the tissue repair and health maintenance [4,5]. The discovery of mesenchymal stem cells that are able to differentiate into solid tissue cells (such as muscles, bones, cartilage and neurons) revealed the new promising cells source for the next revolution in medical science: regenerative medicine. Adult mesenchymal stem cells, also classified as multipotent cells, can be isolated from several tissues and, thereby, represent a potential source for tissue engineering [5,6].
The field of regenerative medicine aims to induce the biological function of specific tissue or organ and repair/replace their structures. The understanding of interactions between stem cells, scaffolds and bioactive molecules, such as growth factors, is considered the triad of tissue engineering - a subfield of regenerative medicine [6,7]. The interplay of stem cells with the growth factors and/or in contact with the unique chemical and morphology surface of biomaterial could induce their differentiation into all cellular lineages. This review will discuss the association of mesenchymal stem cells with biomaterials, such as bioceramics, to replace lost tissue or regenerate damaged tissue or improve the healing mechanisms as a breakthrough of the present implantable grafts technology. In addition, show the current scenario of clinical trials of bone regeneration using mesenchymal stem cells and FDA-approved cell based therapies.
Biomaterial Controlling Stem Cell Fate
The understanding of cell behavior on the surface of biomaterial and the signaling from the surrounding microenvironment, e.g. extracellular matrix (ECM), cells and growth factors, is pivotal to modulate the cell fate through engineering of biomaterial properties. In tissue engineering, the resorbable scaffold is produced to temporarily support the growth of living cells, stimulating physiological interactions through reactions with serum proteins and other cells at molecular level, to recapitulate the normal tissue development process [7,8].
The control of design at macro- and micrometric scale, as well as variations of chemical composition, Nano metric scale topography, surface charge and stiffness matrix has been known to affect the cell activity and the tissue formation. Therefore, biomaterial properties play a key role in tissue engineering and cell-based regenerative therapeutics.
Biomaterial Properties
Immediately after biomaterial placement, its surface becomes completely covered by a thin layer of serum proteins. The first proteins to reach and bind to the biomaterial surface are those present at high concentrations in blood and will further be slowly replaced by proteins with higher affinity with surface. The initial interactions of blood cells and proteins with biomaterial influence clot formation and cell attachment [8]. The molecular conformation of proteins is sensitive to the surface nature. Therefore, surface characteristics determine the availability of specific peptide sequences containing proteins, a phenomenon known to affect cell adhesion and behavior. In contact with blood and biological fluids, hydrophobic surfaces can partially denature proteins, leading to less accessible sites for cellbinding; whereas hydrophilic surfaces promote protein adsorption in a conformation that exposes adhesion sites and enhance cell adhesion [9]. Therefore, a hydrophilic surface, facilitating the cell attachment onto scaffolds, is a prerequisite to biomaterial-cell association, as well as biological performance. The hydrophilicity of scaffolds with the same architecture show that the addition of pristine graphene had a positive impact on cell viability and proliferation, and that enhanced wettability from surface leads to an improved cell response [10]. In other hand, hydrophobic surfaces have been demonstrated to promote the differentiation of stem cells, however with a lower attachment of cells [11,12].
It has been demonstrated that several types of cells is affected by different chemical, morphology and energy surfaces from biomaterial. When manufacturing a scaffold for bone tissue regeneration, the most essential design element is the biocompatibility, where the biomaterial should not demonstrate immunogenicity or elicit an adverse inflammatory response. Furthermore, an ideal scaffold should provide bioactivity (to make direct bonding with living bone), osteoconductivity (to serve as a scaffold for new bone formation), osteoinductivity (recruiting bone-forming cells to induce the new bone formation) and osteogenesis (to provide osteogenic cells to form new bone). In addition, the design of scaffold includes optimal mechanical properties, pore structure and pore size, and a suitable degradation rate for new bone tissue formation [13-16].
A highly-interconnected architecture scaffold, including macro and microporosity, is one of critical factors to vascularization, cells colonization and survival, and supply oxygen and nutrients for transplanted cells [17,18]. A combined macro and micro pore structure of the scaffold have been demonstrated an improvement of cellular responses, such as adhesion, spreading, growth and proliferation, and also increase the bone growth throughout the scaffold. The interconnection of macropores (size > 100 μm) are usually formed by using porogenic agents in bioceramics. It is well defined that porosity should be greater than 100 μm as the requirement for blood vessels and bone tissue ingrowth [17,18]. Several methods already exist to form porous three-dimensional scaffolds with different materials and composites e.g. replica method [19,20], sacrificial phase [19-21], direct foaming [19-22], paste extrusion [23], rapid prototyping [24,25], injection mold [26], slip casting [27], freeze casting [28]. Porous bioactive bioceramics exhibits a chemical bonding with the bone tissue and their interconnected macro and micropores provide a mechanical interlocking and good mechanical behavior in load bearing sites prior to regeneration of new tissue. Long bone defects have shown significantly higher bone ingrowth and torsional strength for cell-seeded scaffolds, when compared to those receiving acellular scaffolds [29,30].
The addition of controlled nanostructures to biomaterial surface has shown promising clinical results when compared to nanosmoother surfaces [31]. It has been established that the interaction between the nanoscale features from biomaterial surface and the biological environment have effects on cell proliferation and differentiation, mediated by alterations in cell adhesion [31]. When associated within a microporosity, the nanoscale features can boost an enhance cell spreading, growth, and gene expression. Although the nanometric surface of biomaterial are several orders of magnitude smaller than stem cells, the interaction occurs through molecular bonding between cell-surface receptors and their ligands, such as integrin receptors in the cells’ focal adhesions that allow the cells to adhere the surface and modulate the sequence of cell events. For understanding the cell behavior on biomaterial surface, studies have shown that cell filopodia and lamellipodia play an important role in the sensing of nanoscale physical and chemical features in the early stages of the cell-surface interaction. The modification of chemical surface of biomaterial is one of the methods to increase wettability and cell adhesion in physiological environment. Controlled and functionalized surface chemistry, such as hydroxyl, amino and carboxyl groups, have been extensively studied by self-assembled monolayers (SAMs) of alkanethiols to mimic the extracellular matrix for cell-related studies and to influence cell-biomaterial surface interaction. A possible mechanism for directing mesenchymal stem cells fate by surface chemical functionality is through the signaling pathways of integrins αv and β1, which are bridges for ECM interactions, transducing signals from the exterior to interior of cells, via signaling pathways [32].
As seen above, besides the biomaterial chemistry and topography influences on cell behavior, the fate of stem cells can also be manipulated by controlling the biomaterial physical properties. Studies demonstrated that differentiation of stem cells can be modulated by elastic modulus (stiffness) of substrates [33-35]. Stiffness is considered the rigidity property of a material, or as the resistance of a solid material to deformation. Although the behavior of different types of cells varies with stiffness of substrate. The increase of cells that migrates towards surfaces of greater stiffness is known as mechanotaxis phenomenon [36]. The osteogenesis culture of human mesenchymal stem cells on uniformly stiff substrates shows more cells spread, larger in area, and stained darker by alkaline phosphatase, indicating a higher expression of the osteogenic marker. In opposite, uniformly soft surfaces indicated minimal staining and, in addition, a significantly higher level of expression of the stem cell marker CD105 was observed, that implies an inhibit of hMSCs osteogenesis [37]. Therefore, the control of triad of properties, surface chemistry, topography and stiffness, has the potential to selectively guide the cell fate within 3D scaffolds [38].
Mesenchymal Stem Cells / Biomaterials association
Most of the surgical procedures aiming to regenerate craniomaxillofacial bone involve the use of either autografts or allografts [39,40]. Despite their importance in the reconstruction of lost or damaged bone structures, there are many limitations, such as cost, limited bone supply and anatomical difficulties [39,41,42]. To surpass these drawbacks, surgeons and scientists have been seeking alternative solutions. There is also evidence that mesenchymal stem cells are immune-modulatory, reducing the expression of inflammatory dendritic cells and suppressing T cells effects [43].
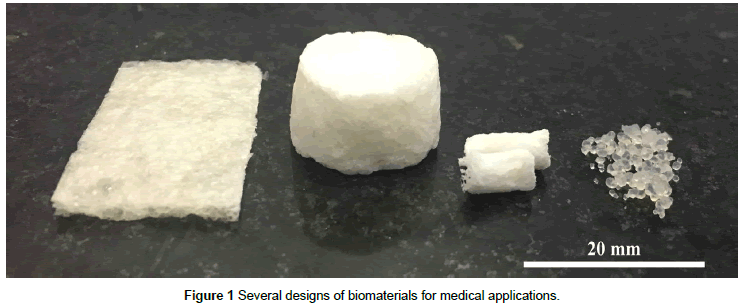
Scaffolds have been fabricated from naturally derived (bone powders, granules and fragments) and synthetic biomaterials (bioceramics, biocompatible polymers, metallic alloys and composites) as three-dimensional bone grafts and bone substitutes [42]. Such materials are supposed to possess osteoconductive properties and lead to the recruitment, proliferation and differentiation of host cells from healthy flanking tissue [44]. In spite of a quick development of a wide diversity of biomaterials with varying physicochemical and structural properties to embody biological characteristics, most empty scaffolds have largely failed to improve bone healing [45,46]. Figure 1 shows some examples of ceramics and/or composite polymer-ceramic such as membrane, scaffolds or microspheres for medical applications.
Biomaterials can be combined with stem cells, delivering transplanted cells to guide the spatially complex process of tissue formation and stimulating osteoconduction, osteoinduction and osteogenesis. In order to both sustain and modulate cell behavior, a scaffold must have biocompatibility, suitable surface texture and chemistry for osteogenic cellular proliferation and differentiation as well as favorable mechanical properties [47,48].
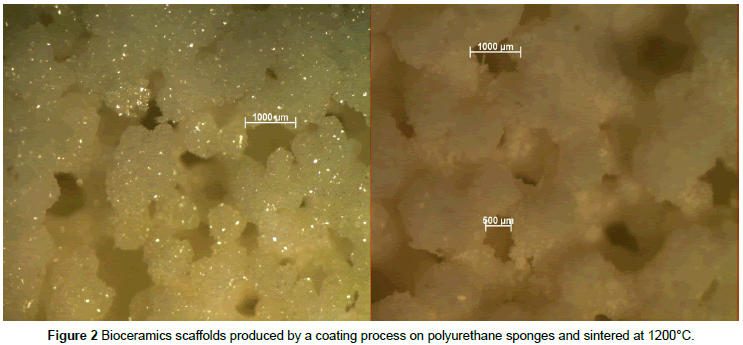
In light of these considerations, ceramics are a family of osteoconductive materials made of calcium phosphate (mainly HA and β-tricalcium phosphate) with advantageous bone binding properties. Ceramics are usually designed to be substitutes for the mineral phase of bone (since 70% of the mineral phase of mature bone is composed of hydroxyapatite) and their in vivo resorption can be modulated by calcium to phosphate ratio and by their porous microstructural configuration [49], as can be seen in Figure 2. As an alternative to ceramics and being the major component of extracellular bone matrix (95% of its organic matrix is composed of type I collagen), collagen is a valuable material for tissue engineering when compared with synthetic polymers since, still being osteoconductive, it provides an attractive surface for cell attachment, besides playing an important role as modulator of cell morphology, cell migration and differentiation [50,51].
Tissue engineering strategies using stem cells aim to improve and increase the prognosis of the grafted materials, using them as carriers for cells with osteogenic properties to achieve proper bone regeneration. Clinical success of these designs ultimately depends on an optimal cell source for bone reconstruction [52]. Almost all craniofacial structures, like bone, cartilage, ligaments and teeth, are derived from mesenchymal cells [53,54] and mesenchymal stem cells (MSCs) have been isolated from most of the post-natal tissues, including several cranio-maxillofacial tissues. [55-57].
Mesenchymal stem cells from the bone marrow niche (BMSCs) have been thoroughly explored in tissue engineering approaches to regenerate bone. Amid bony structures successfully regenerated with BMSCs are calvarial bone in mice, associating BMSCs to gelatin sponges [58], cranial defects of canines, associating BMSCs with β-TCP scaffolds [59], mandibular defects in sheep, combining BMSCs to β-TCP scaffolds [60] and maxillary sinus floor in humans, attaching BMSCs to a biphasic HA/β-TCP scaffold [61]. Due to the scarcity of these cells and since bone marrow harvesting procedure is painful and laborious; there is a great interest in harvesting osteogenic cells from more convenient tissue sources, such adipose tissue-derived stem cells (ASCs) obtained by liposuction or MSCs from deciduous teeth or from teeth being extracted.
Human adipose tissue-derived stem cells (hASCs) can be harvested in abundant quantities from the adipose tissue. It has been suggested that ASCs undergoes osteogenesis with minimal stimulation by exogenous factors, representing a promising option for skeletal tissue engineering trials [62]. Among cranial structures regenerated with ASCs are calvarial defects in mice, associating hASCs to apatite-coated PLGA scaffolds [62], calvarial defects in rats, associating hASCs and PLGA scaffolds [63], calvarial defects in rabbits, seeding rabbit ASCs onto gelatin foam scaffolds [64] and a widespread bilateral calvarial defect in a seven year-old girl using autologous hASCs and fibrin glue [65].
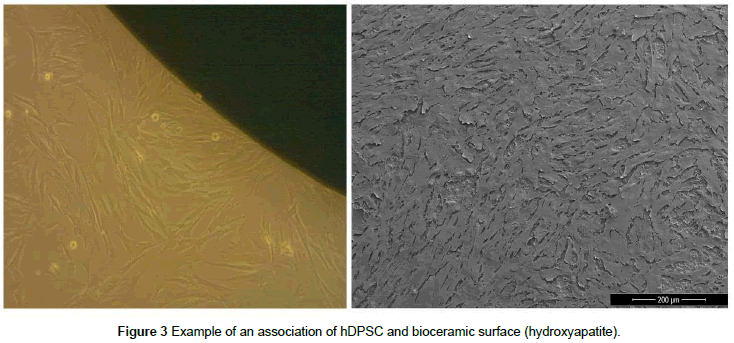
Dental pulp tissue is a promising source of MSCs to be used in tissue bioengineering studies due to their multi differentiation potential, noninvasive and efficient process of isolation, immunosuppressive properties and similarity to osteoblasts [66-68]. Dental pulp stem cells (DPSCs) have also been used to reconstruct large calvarial critical sized defects in rats, attached to a collagen membrane [69,70] and to aid in the regeneration of human mandibular alveolar defects using hDPSCs. An example of protocol to associate hDPSC’s and bioceramics can be performed by simple seeding procedure for an ideal period of cells culture, obtaining an osteoinductive surface. Figure 3 shows a seeding procedure (a) and the FEG-SEM image after 24h of cell culture (b).
Besides displaying in vitro and in vivo multilineage plasticity, dental pulp and adipose tissue are both attractive sources of MSCs for bone tissue engineering for a variety of reasons. ASCs can be easily and promptly isolated from human tissue, besides being readily available in large quantities and it has been reported that ASCs are able to acquire bone cell-like responsiveness and upregulate osteogenic genes in response to shear stress in vitro, which suggests that they are capable to respond to mechanical loads [71]. Moreover, scaffolds loaded with pre-differentiated ASCs cells show more robust bone tissue regeneration when compared to scaffolds seeded with undifferentiated ASCs [64,72-74].
In other hand, DPSCs can be isolated in a noninvasive and highly efficient fashion from deciduous teeth that are usually discarded without ethical concerns [66,75]. Furthermore, they are considered to be analogous to bone cells since they show expression of osteogenic marker proteins as well as response to many growth factors for osteo/odontogenic differentiation [67,76,77] and harbor increased immunosuppressive activity when compared with BMSCs, which could lead to potential advantages in different clinical applications [78]. DPSCs can also be easily cryopreserved for long periods and retain multi differentiation plasticity potential after preservation, which favors the establishment of cryobanks for adult tissue regeneration [78-80].
Methods of Biomaterial-Cell Association
The first step of the traditional tissue engineering approach is, typically, performed by the biomaterial-cells association, seeding differentiated or undifferentiated cells on synthetic scaffolds, developing an ECM in vitro that is crucial in the progression of tissue formation. The cell seeding strategy is a key process that occurs in laboratory and plays a fundamental role in cell distribution within the scaffold. Although the traditional method of static seeding cells onto a scaffold is the most commonly method used, some disadvantages are observed, such as low efficiency of cell seeding and non-uniform cell distribution. In order to surpass these problems, dynamic seeding in an agitated environment or perfusion system allows mechanical conditions for desirable cell density and homogeneity. Bioreactors systems can also provide an enhanced environment for tissue growth, increasing the uniformity of cell seeding and perfusion of three-dimensional cultures. In addition, bioreactor parameters, such as mass transfer, hydrodynamics and environment conditions, could be individually controlled for each desired tissue.
The next step on this association universe is to integrate cells directly on the scaffold fabrication, using a bio-ink for 3-D printing. The development of bio-inks stills a challenge to researchers, once it is composed by living cells, bioactive molecules and biomaterials. Despite the aforementioned challenges the production process has its own complications and increases the difficulties to obtain the desired behavior. The process challenge includes three main steps: cell sedimentation within the ink cartridge; cell viability during extrusion; and cell viability after ink curing; success can be achieved by solving this triad. Some works are aiming on development of new formulations, new molecules and new materials to perfectly combine living cells and bioprinting [81,82]. On the other hand, there are some efforts on the quality analysis of the produced bio-ink, searching on methods to analyze these three main quality aspects [83]. Bioprinting and bio-ink development demonstrates the future of medical devices, clinical applications and diseases studies, as a lot of research efforts are employed, significant advances on patients’ health will become [84].
Clinical Trials and FDA-Approved Therapies
The search on active and completed clinical trials are an indicative for new therapies and where these therapies are based on for future applications, following the logic that is not successful to treat new conditions with old therapies. It was searched on clinicaltrials.gov website [42] with keywords “mesenchymal”, “stem”, “cell”, using the search tool “AND” after the first 3 search keywords and before the term “bone regeneration”, appearing on dialog box as “mesenchymal stem cell AND bone regeneration”. The results were classified on the actual status, not been computed studies with that status: suspended; terminated; withdrawn; and unknown status. It was found 28 studies, which indicates a research effort for evolution on the area of mesenchymal stem cells and biomaterials association for bone regeneration. The results also showed a worldwide spread of clinical trials attempts with some concentration on Europe and Asia, Table 1 demonstrates these results. Another way to indicate the search result is filtrating the conditions treated with these clinical trials. The top twenty conditions are listed below with the number of studies, in Table 2.
| Region Name | Number of Studies |
|---|---|
| World | 28 |
| Asia | 13 |
| Europe | 10 |
| North America | 1 |
| South America | 1 |
| Not Divulged | 3 |
Table 1 Worldwide dispersion of clinical trials.
| Conditions | Number |
|---|---|
| Musculoskeletal Diseases | 7 |
| Wounds and Injuries | 7 |
| Stomatognathic Diseases | 6 |
| Mouth Diseases | 5 |
| Central Nervous System Diseases | 4 |
| Trauma, Nervous System | 4 |
| Arthritis | 3 |
| Bone Diseases | 3 |
| Collagen Diseases | 3 |
| Fractures, Bone | 3 |
| Gingival Diseases | 3 |
| Joint Diseases | 3 |
| Osteoarthritis | 3 |
| Periodontal Diseases | 3 |
| Periodontitis | 3 |
| Rheumatic Diseases | 3 |
| Spinal Cord Diseases | 3 |
| Spinal Cord Injuries | 3 |
| Chronic Periodontitis | 2 |
| Congenital Abnormalities | 2 |
Table 2 Top twenty most cited conditions on clinical trials for mesenchymal stem cell and bone regeneration search.
In addition, the evolution of clinical trials, in some cases, are followed by an attempt to put the innovative therapy available on market, to reach this goal, is necessary to obtain a FDA-approval. On FDA website [43] is listed the attempts of cell based therapies already approved. At the date of this search, there are 14 FDAapproved therapies by OTAT (Office of Tissues and Advanced Therapies) that uses cells, summarized on Table 3, 7 of that are based on hematopoietic stem cells, 1 on fibroblasts, 1 on chondrocytes associated with a porcine collagen membrane for cartilage defects, 1 on keratinocytes and fibroblasts associated with bovine collagen for wound repair and regeneration, 1 on genetically modification of cells for melanoma treatment, 1 on genetically modification of T-cells for acute lymphoblastic leukemia, 1 on peripheral blood mononuclear cells for metastatic castrate resistant prostate cancer and 1 on genetically modification of T-cells for diffuse large B-cells lymphoma. Summarizing, from 14 available cell based therapies approved by FDA 2 of them are a direct association of cells and biomaterials.
| Trade name | Manufacturer | Indication |
|---|---|---|
| ALLOCORD | SSM Cardinal Glennon Children's Medical Center | For use in unrelated donor hematopoietic progenitor cell transplantation procedures in conjunction with an appropriate preparative regimen for hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system that are inherited, acquired, or result from myeloablative treatment. |
| CLEVECORD | Cleveland Cord Blood Center | For use in unrelated donor hematopoietic progenitor cell transplantation procedures in conjunction with an appropriate preparative regimen for hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system that are inherited, acquired, or result from myeloablative treatment. |
| HEMACORD | New York Blood Center, Inc. | For use in unrelated donor hematopoietic progenitor cell transplantation procedures in conjunction with an appropriate preparative regimen for hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system that are inherited, acquired, or result from myeloablative treatment. |
| DUCORD | Duke University School of Medicine | For use in unrelated donor hematopoietic progenitor cell transplantation procedures in conjunction with an appropriate preparative regimen for hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system that are inherited, acquired, or result from myeloablative treatment. |
| NONE | Clinimmune Labs, University of Colorado Cord Blood Bank | For use in unrelated donor hematopoietic progenitor cell transplantation procedures in conjunction with an appropriate preparative regimen for hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system that are inherited, acquired, or result from myeloablative treatment. |
| NONE | LifeSouth Community Blood Centers, Inc. | For use in unrelated donor hematopoietic progenitor cell transplantation procedures in conjunction with an appropriate preparative regimen for hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system that are inherited, acquired, or result from myeloablative treatment. |
| NONE | Bloodworks | For use in unrelated donor hematopoietic progenitor cell transplantation procedures in conjunction with an appropriate preparative regimen for hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system that are inherited, acquired, or result from myeloablative treatment. |
| AZFICEL-T | Fibrocell Technologies, Inc. | Indicated for improvement of the appearance of moderate to severe nasolabial fold wrinkles in adults. |
| MACI | Vericel Corporation | Indicated for the repair of single or multiple symptomatic, full-thickness cartilage defects of the knee with or without bone involvement in adults. MACI is an autologous cellularized scaffold product. |
| GINTUIT | Organogenesis Incorporated | Is an allogeneic cellularized scaffold product indicated for topical (non-submerged) application to a surgically created vascular wound bed in the treatment of mucogingival conditions in adults. |
| IMLYGIC | Amgen Inc. | Indicated for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma recurrent after initial surgery. |
| KYMRIAH | Novartis Pharmaceuticals Corporation | Indicated for the treatment of patients up to 25 years of age with B-cell precursor acute lymphoblastic leukemia (ALL) that is refractory or in second or later relapse. |
| PROVENGE | Dendreon Corporation | For the treatment of asymptomatic or minimally symptomatic metastatic castrate resistant (hormone refractory) prostate cancer. |
| YESCARTA | Kite Pharma, Incorporated | A CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B-cell lymphoma, high grade B-cell lymphoma and DLBCL arising from follicular lymphoma. |
Table 3 FDA-approved to date cell based therapies.
The recent research effort, demonstrated on clinical trials attempts, illustrates a promising scenario about to treat new conditions with new therapies technology and new approaches. This research effort is being validating when some researches acquires FDA approval to make the therapy technology available to society, improving life quality of patients.
Conclusion
The use of adult stem cells for bone regeneration is a robust therapeutic option worldwide, being spread in clinical trials. FDAapproved cell based therapies also indicates the safety and promising area evolution that evokes the established approach of new therapies to treat new conditions. The understanding and optimization of settings to ideally match biomaterial scaffolds with stem cells harboring higher degrees of osteogenic potential, will contribute to make stem cell-based therapies a better bid than the golden standard autologous bone substitute for reconstruction and rehabilitation of craniofacial osseous defects.
Acknowledgement
The authors would like to acknowledge Military Institute of Engineering (IME) and R-Crio Criogenia S/A for the support. The authors declare no conflict of interest related to this study.
References
- McKeown RE (2009) The epidemiologic transition: Changing patterns of mortality and population dynamics. Am J Lifestyle Med 3:19-26.
- Dupont KM, Sharma K, Stevens HY, Boerckel JD, García AJ, et al. (2010) Human Stem cell delivery for treatment of large segmental bone defects. Proc Natl Acad Sci U S A 107: 3305-3310.
- Ullah I, Subbarao RB, Rho GJ (2015) Human mesenchymal stem cells - Current trends and future prospective. Biosci Rep 35: e00191.
- Anderson HJ, Sahoo JK, Ulijn RV, Dalby MJ (2016) Mesenchymal stem cell fate: Applying biomaterials for control of stem cell behavior. Front Bioeng Biotechnol 4: 38.
- Waese EYL, Kandel RR, Stanford WL (2008) Application of stem cells in bone repair. Skeletal Radiol 37: 601-608.
- Langer R, Vacanti JP (1993) Tissue engineering. Science 260: 920-926
- Katari R, Peloso A, Orlando G (2015) Tissue engineering and regenerative medicine: Semantic considerations for an evolving paradigm. Front Bioeng Biotechnol 57: 1-6.
- Xu LC, Bauer J, Siedlecki C (2014) Proteins, platelets and blood coagulation at biomaterial interfaces. Colloids Surfaces B Biointerfaces 124: 49-68.
- Gittens RA, Scheideler L, Rupp F, Hyzy SL, Geis-Gerstorfer J, et al. (2014) A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomaterialia 10: 2907-2918.
- Wang W, Caetano G, Ambler WS, Blaker JJ, Frade MA (2016) Enhancing the hydrophilicity and cell attachment of 3D printed PCL/graphene scaffolds for bone tissue engineering. Materials 9: 992.
- Valamehr B, Jonas SJ, Polleux J, Qiao R, Guo S, et al. (2008) Hydrophobic surfaces for enhanced differentiation of embryonic stem cell-derived embryoid bodies. Proc Natl Acad Sci U S A 105: 14459-14464.
- Gandhimathi C, Venugopal J, Ravichandran R, Sundarrajan S, Suganya S, et al. (2013) Mimicking nanofibrous hybrid bone substitute for mesenchymal stem cells differentiation into osteogenesis. Macromol Biosci 13: 696-706.
- De Sá KD, Figueira DR, Miguel SP, Correia TR, Silva AP, et al. (2017)3D scaffolds coated with nanofibers displaying bactericidal activity for bone tissue applications. Inter J Poly Mater 66: 9.
- Luo Z, Yang Y, Deng Y, Sun Y, Yang H, et al. (2016) Peptide-incorporated 3D porous alginate scaffolds with enhanced osteogenesis for bone tissue engineering. Colloids Surf B Biointerfaces 143: 243-251.
- Zhu X, Liu N, Yaszemski MJ, Lu L (2010) Effects of composite formulation on mechanical properties of biodegradable poly(propylene fumarate)/bone fiber scaffolds. Int J Polym Sci, pp: 1-6.
- Milovac D, Ferrer GG, Ivankovic M, Ivankovic H (2014) PCL-coated hydroxyapatite scaffold derived from cuttlefish bone: Morphology, mechanical properties and bioactivity In Mater. Sci Eng C Mater Biol Appl 34: 437-445.
- You C, Lee MH, Lee HJ, Han MH, TY Kwon, et al. (2017) The effect of macro/micro combination pore structure of biphasic calcium phosphate scaffold on bioactivity. In Ceramics International 43: 3540-3546.
- Kim JW, Shin KH, Koh YH, Hah MJ, Moon J, et al. (2017) Production of poly (ε-caprolactone)/hydroxyapatite composite scaffolds with a tailored macro/micro-porous structure, high mechanical properties and excellent bioactivity materials. 10: 1123.
- Studart AR, Gonzenbach UT, Tervoort E (2006) Processing routes to macroporous ceramics: A review. J Am Ceram So 89: 1771-1789.
- Schwartzwalder K, Somers AV (1963) Methods of making porous ceramic articles. US patent.
- Liu DM (1998) Preparation and characterization of porous hydroxyapatite bioceramic via a slip-casing route. Ceram Int 24: 441-446.
- Gonzenbach UT, Studart AR, Tervoort E (2007) Macroporous ceramics from particles stabilized wet foams. J Am Ceram Soc 90: 16-22.
- Aneziris CG , Schärfl W, Ullrich B (2007) Microstructure evaluation of Al2O3 ceramics with Mg- PSZ- and TiO2-additions. J Eur Ceram Soc 27: 3191-3199.
- Tay BY, Evans JRG, Edirsinghe MJ (2003) Solid free form fabrication of ceramics. Int Mater Rev 48: 341-370.
- Seitz H, Rieder W, Irsen S (2005) Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res B 74: 782-788.
- Marçal RLSB (2016) Biomaterials produced by injection molding: Reference module in materials science and materials engineering. Oxford, Elsevier.
- Marçal RLSB, Navarro da Rocha D, Prado da Silva MH (2017) Slip casting used as a forming technique for hydroxyapatite processing, key engineering materials. Bioceramics 720: 219-222.
- Lee H, Jang TS, Song J, Kim HE, Jung HD (2017) The production of porous hydroxyapatite scaffolds with graded porosity by sequential freeze-casting. Materials (Basel) 10: 367.
- Dupont KM, Sharma K, Stevens HY, Boerckel JD, García AJ, et al. (2010) Human stem cell delivery for treatment of large segmental bone defects. Proc Natl Acad Sci 107: 3305-3310.
- Pilia M, Guda T, Appleford M (2013) Development of composite scaffolds for load-bearing segmental bone defects. BioMed Res Int 1-15.
- Dalby MJ, Gadegaard N, Oreffo ROC (2014) Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat Mater 13: 558-569.
- Hao L, Fu X, Li T, Zhao N, Shi X, et al. (2016) Surface chemistry from wettability and charge for the control of mesenchymal stem cell fate through self-assembled monolayers. Colloids Surf B Biointerfaces 148: 549-556.
- Engler AJ, Sen S, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell. 126: 677-689.
- Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, et al. (2009) Substrate stiffness affects early differentiation events in embryonic stem cells. Eur Cell Mater 18:1-13.
- Her GJ, Wu HC, Chen MH, Chen MY, ChangSC, et al. (2013) Control of three-dimensional substrate stiffness to manipulate mesenchymal stem cell fate toward neuronal or glial lineages Acta Biomaterialia 9: 5170-5180.
- Wells RG (2008) The role of matrix stiffness in regulating cell behavior. Hepatology 47: 1394-1400.
- Yang C, DelRio FW, Ma H, Killaars AR, Basta LP, et al. (2016) Spatially patterned matrix elasticity directs stem cell fate. Proc Natl Acad Sci U S A 113: 4439-4445.
- Najdanović JG, Cvetković VJ, Stojanović S, Vukelić-Nikolić MD, Čakić-MiloševićMM, et al. (2006) Effects of bone tissue engineering triad components on vascularization process: comparative gene expression and histological evaluation in an ectopic bone-forming model. Biotechnol Biotechnol Equip 30: 1122-1131.
- Stevens B, Yang Y, Mohandas A, Stucker B, Nguyen KT (2008) A review of materials, fabrication methods, and strategies used to enhance bone regeneration in engineered bone tissues. J Biomed Mater Res B Appl Biomater 85: 573-582.
- Sen MK, Miclau T (2007) Autologous iliac crest bone graft: should it still be the gold standard for treating non-unions? Injury 38 75-80.
- Goulet JA, Senunas LE, De Silva GL, Greenfield ML (1997) Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res 339: 76-81.
- Laurencin C, Khan Y, El-Amin SF (2006) Bone graft substitutes. Expert Rev Med Devices 3: 49-57.
- Murphy MB, Moncivais K, Caplan AI (2013) Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 45: 54
- Zuk PA (2008) Tissue engineering craniofacial defects with adult stem cells? Are we ready yet? Pediatr Res 63: 478-486.
- Szpalski C, Wetterau M, Barr J, Warren SM (2012) Bone tissue engineering: Current strategies and techniques part I-scaffolds. Tissue Eng Part B Rev 18: 246-257.
- Petite H, Viateau V, Bensaïd W, Meunier A, de Pollak C, et al. (2000) Tissue-engineered bone regeneration. Nat Biotechnol 18: 959-963.
- Khan WS, Rayan F, Dhinsa BS, Marsh D (2012) An osteoconductive, osteoinductive, and osteogenic tissue-engineered product for trauma and orthopaedic surgery: How far are we? Stem Cells Int 12: 7.
- Yuan H, Fernandes H, Habibovic P, de Boer J, Barradas AMC, et al. (2010) Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc Natl Acad Sci U S A 107: 13614–13619.
- Sahoo NG, Pan YZ, Li L, He CB (2013) Nanocomposites for bone tissue regeneration. Nanomedicine 8: 639-653.
- Böhm S, Strauß C, Stoiber S, Kasper C, Charwat V (2017) Impact of source and manufacturing of collagen matrices on fibroblast cell growth and platelet aggregation. Materials 10: 1086.
- Dozza B, Lesci IG, Duchi S, Della Bella E, Martini L, et al. (2017) When size matters: Differences in demineralized bone matrix particles affect collagen structure, mesenchymal stem cell behavior and osteogenic potential. J Biomed Mater Res Part A 105A: 1019-1033.
- Waese EY, Kandel RA, Stanford WL (2008) Application of stem cells in bone repair. Skeletal Radiol 37: 601-608
- Zhao H, Chai Y (2015) Stem cells in teeth and craniofacial bones. J Dent Res 94:1495-1501.
- Porada CD, Zanjani ED, Almeida-Porad G (2006) Adult mesenchymal stem cells: A pluripotent population with multiple applications. Curr Stem Cell Res Ther 1: 365-369.
- Abu Kasim NH, Govindasamy V, Gnanasegaran N, Musa S, Pradeep PJ, et al. (2015) Unique molecular signatures influencing the biological function and fate of post-natal stem cells isolated from different sources. J Tissue Eng Regen Med 9: 252-266.
- Pirjali T, Azarpira N, Ayatollahi M, Aghdaie MH, Geramizadeh B, et al. (2013) Isolation and characterization of human mesenchymal stem cells derived from human umbilical cord Wharton's jelly and amniotic membrane. Int J Organ Transplant Med 4: 111-116.
- Alhadlaq A, Mao JJ (2004) Mesenchymal stem cells: Isolation and therapeutics. Stem Cells Dev13: 436-448.
- Zou D, Zhang Z, Ye D, Tang A, Deng L, et al. (2011) Repair of critical-sized rat calvarial defects using genetically engineered bone marrow-derived mesenchymal stem cells overexpressing hypoxia-inducible factor-1α. Stem Cells 29: 1380-1390.
- Umeda H, Kanemaru S, Yamashita M, Kishimoto M, Tamura Y, et al. (2007) Bone regeneration of canine skull using bone marrow-derived stromal cells and beta-tricalcium phosphate. Laryngoscope 117: 997-1003.
- Nolff MC, Gellrich NC, Hauschild G, Fehr M, Bormann KH, et al. (2009) Comparison of two beta-tricalcium phosphate composite grafts used for reconstruction of mandibular critical size bone defects. Vet Comp Orthop Traumatol 22: 96-102.
- Shayesteh YS, Khojasteh A, Soleimani M, Alikhasi M, Khoshzaban A, et al. (2008) Sinus augmentation using human mesenchymal stem cells loaded into a beta-tricalcium phosphate/hydroxyapatite scaffold. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106: 203-209.
- Levi B, James AW, Nelson ER, Vistnes D, Wu B, et al. (2010) Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One 5: e11177.
- Yoon E, Dhar S, Chun DE, Gharibjanian NA, Evans GR (2007) In vivo osteogenic potential of human adipose-derived stem cells/poly lactide-co-glycolic acid constructs for bone regeneration in a rat critical-sized calvarial defect model. Tissue Eng 13: 619-627.
- Dudas JR, Karra KG, Cooper GM, Penascino VM, Mooney MP, et al. (2006) The osteogenic potential of adipose-derived stem cells for the repair of rabbit calvarial defects. Ann Plast Surg 56: 543-548.
- Lendeckel S, Jödicke A, Christophis P, Heidinger K, Wolff J, et al. (2004) Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg 32: 370-373.
- Bakopoulou A, Apatzidou D, Aggelidou E, Gousopoulou E, Leyhausen G, et al. (2017) Isolation and prolonged expansion of oral mesenchymal stem cells under clinical-grade, GMP-compliant conditions differentially affects “stemness” properties. Stem Cell Res Ther 8: 247.
- Yang H, Li J, Sun J, Guo W, Li H, et al. (2017) Cells isolated from cryopreserved dental follicle display similar characteristics to cryopreserved dental follicle cells. Cryobiology. 78: 47-55.
- Rodas-Junco BA, Villicaña C (2017) Dental pulp stem cells: Current advances in isolation, expansion and preservation. J Tissue Eng Regen Med 14: 333-347.
- Bueno DF, Kerkis I, Costa AM, Martins MT, Kobayashi GS, et al. (2009) New source of muscle-derived stem cells with potential for alveolar bone reconstruction in cleft lip and/or palate patients. Tissue Eng Part A. 15: 427-435.
- Costa AM, Bueno DF, Martins MT, Kerkis I, Kerkis A, et al. (2008) Reconstruction of large cranial defects in non-immunosuppressed experimental design with human dental pulp stem cells. J Craniofac Surg. 19: 204-210.
- Knippenberg M, Helder MN, Doulabi BZ, Semeins CM, Wuisman PI, et al. (2005) Adipose tissue-derived mesenchymal stem cells acquire bone cell-like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Eng 11: 1780-1788.
- Di Bella C, Farlie P, Penington AJ (2008) Bone regeneration in a rabbit critical-sized skull defect using autologous adipose-derived cells. Tissue Eng Part A 14: 483-490.
- Liu B, Cui L, Liu GP, Cao YL, Zhu JT, et al. (2009) Tissue-engineering bone with ADSCs and coral scaffold for repairing of cranial bone defect in canine. Zhonghua Zheng Xing Wai Ke Za Zhi 25: 204-208.
- Cui L, Liu B, Liu G, Zhang W, Cen L, et al. (2007) Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials. 28: 5477-5486.
- Huang GT, Gronthos S, Shi S(2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J Dent Res. 88: 792-806.
- Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, et al. (2006) Craniofacial tissue engineering by stem cells. J Dent Res 85: 966-979.
- Liu J, Jin T, Chang S, Ritchie HH, Smith AJ, et al. (2007) Matrix and TGF-beta-related gene expression during human dental pulp stem cell (DPSC) mineralization. In Vitro Cell Dev Biol Anim 43: 120-128.
- Saez DM, Sasaki RT, Neves AC, da Silva MCP (2016) Stem cells from human exfoliated deciduous teeth: A growing literature. Cells Tissues Organs 202: 269-280.
- Graziano A, Biunno I, De Blasio P, Giordano A (2007) The tissue banking in cancer and stem cell research. J Cell Physiol 212: 345-347.
- Lindemann D, Werle SB, Steffens D, Garcia-Godoy F, Pranke P, et al. (2014) Effects of cryopreservation on the characteristics of dental pulp stem cells of intact deciduous teeth. Arch Oral Biol 59: 970-976.
- Demirtaş TT, Irmak G, Gümüşderelioğlu M (2017) A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 9: 1-12
- Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, et al. (2010) Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2: 1-14.
- Dubbin K, Tabet A, Heilshorn SC (2017) Quantitative criteria to benchmark new and existing bio-inks for cell compatibility. Biofabrication 9: 1-11.
- Donderwinkel I, van Hest JCM, Cameron NR (2017) Bio-inks for 3D bioprinting: Recent advances and future prospects. Polym Chem 8: 4451-4471.