Review Article, Biomater Med Appl Vol: 1 Issue: 1
Novel Biocompatible Ultrananocrystalline Diamond Coating Technology for a New Generation of Medical Implants, Devices, and Scaffolds for Developmental Biology
1Department of Materials Science and Bioengineering, University of Texas at Dallas, USA
2Advanced Diamond Technologies, Illinois, USA
3Original Biomedical Implants (OBI-USA and OBI-México)
*Corresponding Author : Orlando Auciello
Department of Materials Science and Bioengineering, University of Texas at Dallas, USA
Tel: 972-883-4731
Fax: 972-883- 5725
E-mail: Orlando.auciello@utdallas.edu
Received: August 11, 2017 Accepted: September 18, 2017 Published: September 27, 2017
Citation: Auciello O (2017) Novel Biocompatible Ultrananocrystalline Diamond Coating Technology for a New Generation of Medical Implants, Devices, and Scaffolds for Developmental Biology. Biomater Med Appl 1:1.
Abstract
A new biomaterial paradigm for a new generation of implantable medical devices, prostheses, and scaffolds for stem cells growth and differentiation for developmental biology is provided by a novel multifunctional/biocompatible/body fluid corrosion-resistant ultra-nanocrystalline diamond (UNCD) film developed and patented in recent years. The UNCD coating provides a unique combination of multi functionalities, including biocompatibility, bioinertnes to chemical attack by body fluids, and surface chemistry, based on Carbon (C) atoms (element of life), to enable a new generation of implantable medical devices, prostheses, and scaffolds for tissue engineering, to impact the quality of life of people. This review article includes the following topics: 1) Brief description of the synthesis and properties of UNCD films and comparison with other diamond film technologies. 2) Brief description of key medical devices and prostheses under development, based on UNCD coatings. 3) Demonstration of UNCD as an outstanding scaffold for growing stem cells and induces differentiation for developmental biology, to treat specific medical conditions.Topics discussed include: • UNCD-coated microchip (artificial retina) is implantable on the eye’s retina to restore partial vision to people. Who are blind by retinitis pigmentosa, produced by genetically induced degeneration of the retina photoreceptors (The Argus II device, developed by a team of researchers and doctors from universities, national laboratories and the second Sight was currently implanted commercially in the USA and Europe,and it was selected by TIME magazine as one of the top 25 inventions for 2013). • UNCD- coated intraocular device for the treatment of glaucoma (intraocular pressure associated optical neuropathy that could lead to blindness). • UNCD-coated metal dental implants with order of magnitudelonger life and superior performance than current implants. • UNCD- coated prostheses (hips, knees and other metal-basedimplants); • New generation of Li-ion batteries (LIBs) for defibrillator/ pacemakers, with ≥ 10 × longer life and safer than current LIBs. • Description of UNCD-based scaffolds
Keywords: Ultrananocrystalline diamond (UNCD); Coating; Encapsulated Si chip (artificial retina); Coated dental implants; Glaucoma drainage devices; Prostheses
Introduction
The novel ultrananocrystalline diamond (UNCD) coating technology, developed by the author of this review article and many colleagues [1] and patented [2-5], in recent years, is providing a new paradigm material for a new generation of implantable medical devices, prostheses, and new scaffolds for stem cell growth and differentiation for developmental biology. It is important to point out that the UNCD coating technology is already in the market, commercialized by Advanced Diamond Technologies, a company cofounded by O. Auciello and J.A. Carlisle in 2003, profitable in 2014). Products in the market include
a) Low manufacturing cost/high performing UNCD coatings for mechanical pumps seals and bearings, with order of magnitude lower coefficient of friction (0.02 - 0.04) than any other material (≥ 0.3) previously used, thus providing up to 20% savings in cost of energy of running the mechanical pumps because much less torque needed.
b) Electrically conductive Boron-doped UNCD coating to cover metal electrodes from corrosion in a new generation of water purification systems for domestic and industrial uses, based on electrolysis, to create ozone that kills all bacteria in the water and destroy any other contaminant.
In the field of application to medical technologies, the UNCD coating exhibits a unique combination of multifunctionalities and properties, including biocompatibility, bio-inertness to chemical attack by body fluids and unique surface chemistry, enabling new generations of implantable prostheses, medical devices and scaffolds for tissue engineering.
Biomedical products under development include:
1) UNCD- coated microchip, implantable inside the human eye, to restore partial vision to people blind by retinitis pigmentosa , produced by genetically-induced death of retina photoreceptors (1st generation device (2009 R&D and Editor’s Choice Award) the first generation device (Argus II) without the UNCD coating yet, is currently marketed by Second Sight (CA, USA) restoring partial vision to blind people (selected as a top invention in 2013 by TIME magazine).
2) UNCD-coated intraocular device for treatment of Glaucoma
3) UNCD-coated metal dental, hips, knees and other prostheses with ≥ 10 × longer life and superior performance than current implants.
4) Li-ion batteries with electrically conductive UNCD-coated electrodes and membranes and inner walls of metal LIB’s cases, providing ≥ 10 × longer life and safer, for new defibrillator/pacemakers with order of magnitudes longer life without changing batteries, as it occurs with theses devices powered by current LIB’s.
Materials and Methods
Synthesis and Structure of biocompatible UNCD films and comparison with other diamond films
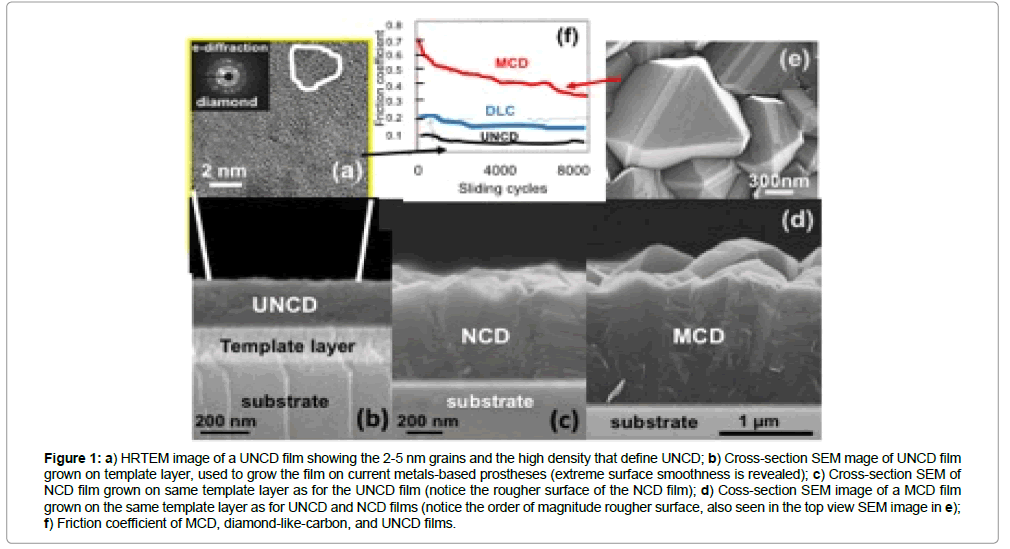
Several types of diamond thin films have been and are currently being grown on surfaces of insulators, semiconductors and metals based materials used in many devices and components of technological products, and the properties studied systematically. The diamond films produced until now exhibit different microstructure, surface morphology, and properties. Diamond films are grown by many groups worldwide, using mainly microwave plasma enhanced chemical vapor deposition (MPCVD) [1-6] or hot filament chemical vapor deposition (HFCVD) processes, in the research laboratory environment [7-10] and for fabrication of products based on diamond coatings [11,12], in conjunction with a wet-chemical-diamondnanoparticles “seeding” process. the conventional diamond film growth process via MPCVD and HFCVD, involves “seeding”. The surface of the substrate with micro or nanocrystalline diamond particles by immersing the substrate in a solution of methanol and diamond particles in a sonicator, which sounds waves, shake the particles and embed them on the surface as “seeds” to nucleate and induce the growth of diamond film. For the MPCVD method, a mixture of gases, containing CHM4 molecules, as the main species, is inserted in an air evacuated chamber and microwave power coupled to the gas to produce a plasma involving ionized and neutral atoms and molecules containing C, H and other components such as Ar, and electrons [1-6]. For the HFCVD process, an array of hot filaments (heated at ~ 2200˚C) induce cracking of CH4 molecules impacting on the hot surface, producing radicals that induce growth of diamond films [7-12]. Use of a hydrogen-rich chemistry [H (balance)/CH4 (0.1 to 4%)] [1,6] results in microcrystalline diamond (MCD) (1-5 μm grains and columnar microstructure) (for ~ 1% CH4) and Nano crystalline diamond (NCD) (10-100 nm grains) (for up to ~ 4% CH4) films. The H/CH4 chemistry-based growth process is driven by CHx (x = 2-3) radical’s interaction with the substrate surface, involving hydrogen abstraction process, ultimately resulting in carbon atoms bonding into positions corresponding to the diamond lattice. A graphitic phase that co-deposits with the diamond phase is etched by chemical reaction of the carbon sp2 bound atoms with atomic hydrogen in the plasma, which also etches the diamond phase, although at ~ 50 times lower rate than for graphite, which results in formation of inter-granular voids and columnar large grains ( ≥ 1 μm). The MCD films exhibit high compressive stress, poor inter-granular adhesion, and rough surfaces (Figure 1), which are not suitable for coating prostheses, requiring smooth (Figure 1) low friction surfaces of coatings, as provided by the UNCD coating (Figure 1) [1]. Therefore, MCD films are not well suited for application to the medical devices and implants discussed in this article [1]. Diamond films grain size can be reduced to 10-100 nm, characteristic of NCD films, by increasing the CH4/H2 ratio in the plasma, resulting in relatively smooth NCD films (Figure 1), [1-6] but with increased non-diamond components in the grain boundaries (Figure 1).
Figure 1: a) HRTEM image of a UNCD film showing the 2-5 nm grains and the high density that defines UNCD; b) Cross-section SEM mage of UNCD film grown on template layer, used to grow the film on current metals-based prostheses (extreme surface smoothness is revealed); c) Cross-section SEM of NCD film grown on same template layer as for the UNCD film (notice the rougher surface of the NCD film); d) Coss-section SEM image of a MCD film grown on the same template layer as for UNCD and NCD films (notice the order of magnitude rougher surface, also seen in the top view SEM image in e); f) Friction coefficient of MCD, diamond-like-carbon, and UNCD films.
Figure 1 shows clearly why UNCD coatings are far superior than MCD and NCD coatings for prostheses and other implantable devices, and even far superior than DLC films, currently used as coating for some prostheses.
Differently to MCD and NCD films, the UNCD films discussed in this article are produced by microwave MPCVD [1] and HFCVD, using a novel [2-5] argon-rich chemistry [Ar (99%)/CHM4(1%)] [1] which produces carbon dimers (C2) and CH radicals in the plasma, from methane decomposition, via 2CH4 → C2H2 + 3H2 and C2H2 → C2 + H2 chemical reactions.
Initial experimental and theoretical work indicated that C2 dimers play a critical role in the UNCD film nucleation and growth [1,13] because C2 dimers have low activation energy (~ 6 kcal/ mol) for insertion into the substrate surface, thus establishing the nucleation characteristics of UNCD films. Modeling [14], subsequent to the first one [13] suggested that while the C2 population in the plasma is high, the population near the surface may be low, and other hydrocarbon radicals (e.g., CH3, C2H2) are also substantial or the main contributors to the UNCD film growth [14]. However, this model [14] did not fully explain the low temperature growth (≤ 400˚C) of UNCD films. Clearly, further experimental and theoretical work is need. In any case, the uniqueness of UNCD films is that they exhibit a nanostructure with the smallest grain size observed today (Figure 1a) for diamond films. It is also relevant that insertion of nitrogen in the gas mixture (e.g. Ar (79 sccm)/CH4(1 sccm)/N2 (20 sccm) results in UNCD films, named N-UNCD, with ~ 10 nm grain size and ~ 2 nm wide gran boundaries [2,15], which exhibit good electrical conductivity, via nitrogen incorporation into the grain boundaries, satisfying dangling C bonds, and providing electrons for electrical conduction.
A critical outcome of the UNCD nucleation and growth processes is that it produces diamond films at the lowest temperatures (350- 400˚C) demonstrated today. This unique property of UNCD coatings enables encapsulation of Silicon (Si)-based microchips for implantation in the human body, as demonstrated by the UNCD coating encapsulation of a Si microchip implantable inside the eye [16], as part of an artificial retina to restore partial vision to people blind by retinitis pigmentosa. Low temperature UNCD film growth on CMOS devices may provide the bases for monolithically integrated UNCD-MEMS/NEMS/CMOS devices [17] for biosensors and MEMS-drug deliver devices implantable in the human body.
In relation to the synthesis of UNCD films, a process named bias enhanced nucleation/bias enhanced growth (BEN/BEG), first demonstrated for the MPCVD to nucleate diamond particles on a substrate surface,[18] was more recently demonstrated suitable for growing dense UNCD to MCD films, over large areas (up to 200 mm diameter)[1]. The BEN/BEG process was also developed for the HFCVD process as well [19-21]. The latter being scaled up now to cover large areas [21] for commercialization of the process. The BENBEG process involves biasing the substrate with a negative voltage (~ -150-300 V) to accelerate C+ and CHx+ ions from the plasma towards the substrate’s surface, where they get implanted producing a carbide layer, which nucleates and induce grow of UNCD or any other polycrystalline diamond films [1,19-23]. BEN/BEG provides several advantages over conventional UNCD growth with “wet” chemical diamond seeding, as discussed above, namely: a) comparable or better seeding efficiency, b) stronger adhesion to substrates, due to the energetic ions sub-plantation in the sub-surface region and c) an integrated fully dry nucleation/growth process using plasmas only. BEN/BEG processes, using H2/CH4 chemistries produced NCD (30-100 nm grains) films [24]. However, this process resulted in the formation of diamond clusters, relatively high surface roughness, high compressive stress, film delamination, and high non-diamond phase content [24]. More recently, our group developed a low pressure MPCVD BEN/BEG process, using the Ar/CH4 chemistry, which yielded UNCD films with the UNCD nanostructure (Figure 1a), low stress (~ 80-100 MPa), smooth surfaces (rms ~ 4-6 nm), and higher growth rates (~ 1 μm/hr.) [1,21] than for UNCD films grown without bias (~ 02-0.3 μm/h) [1]. BEN/BEG UNCD films may provide the most appropriate process for coating of dental and other prosthesis implants, as discussed in this article.
Results
Properties of UNCD films relevant to medical implants and devices
Properties of UNCD films, relevant to the development of the medical implants and devices discussed in this article include:
a) Hardness (98 GPA) and Young’s modulus (980 GPA) [1] close to corresponding values for single crystal diamond (100 GPA and 1200 GPA, respectively)
b) The lowest coefficient of friction (0.02 -0.04) [1] of any biocompatible coating known today
c) high fracture strength (~ 5.3 GPA);[1] d) relatively high electrical conductivity by incorporation of N atoms in the grain boundaries (N-UNCD) [1]. With respect to this property, Garret et al. recently demonstrated excellent performance of N-UNCD coated metallic electrodes for neural stimulation [25]. An alternative process that was developed to produce electrically conductive UNCD coatings is by inserting B atoms substituting C atoms in the diamond lattice and supplying electrons to the conduction band, to produce what is known now as electrically conductive B-UNCD films [26]
d) Extreme resistance to chemical attack by body fluids, as demonstrated by UNCD-coated Si microchip implanted inside rabbit eyes as main component of an artificial retina to restore partial sight to people blind by genetically-induced degeneration of photoreceptors [16]
e) Tailoring of UNCD surface chemistry to use UNCD films as scaffolds for efficient stem cell growth and differentiation for developmental biology [27] in this sense, the demonstration that biological cells can grow efficiently on the surface of UNCD coatings [27] may induce enhanced grafting of UNCD-coated dental and other prostheses, via bone cell growth onto the UNCD surface of the implant (currently under investigation by our group).
UNCD coatings on implantable medical devices and prostheses: comparison with alternative biocompatible coatings
Devices for restoring vision to blind people background: Human vision results from photons penetrating the eye and exciting the retina photoreceptors. The photonic excitation transforms into electrical charges, which excite the bipolar cells connected to the photoreceptors. The bipolar cells operate amplifying the electrical pulses produced by the carriers. These charges were injected into the ganglion cells, which transmit the charges through their axons, forming the optical nerve, to the brain, where the images are finally formed.
R&D performed during several recent years focused on developing implantable devices for neural stimulation and hybrid bionic systems to restore lost human motor and sensory functions. First generation of neural prostheses are being used to restore hearing and sight, via cochlear implants and artificial retina (the Argus II device, marketed by Second Sight, is the first device worldwide being implanted in people blind by retinitis picmentosa, who regain partial vision), respectively [28,29].
UNCD-encapsulated silicon microchip: main component of artificial retina to restore partial vision to blind people
Background on technologies for vision restoration: Several vision restoration technologies have been and are being explored. A review of those technologies can see in a recent article by Auciello et al. [30], and will not be repeated here. The focus of this part of the article is on briefly describing the R&D performed to develop the Argus II Retinal Prosthesis [31]. This device involves a Si-base microchip that in the final rendition should be implanted inside the eye on the ganglion cell layer (schematic in 2 left side), receive images, wireless, from a camera on glasses and inject processed electrical pulses to the ganglion cells through a large electrode array, finally transmitted to the brain via the ganglion cell axons bundle (optical nerve) [31]. The Argus II device was developed by a team of researchers from Universities, National Laboratories, and Second Sight (company currently commercializing the device, during a ten years Department of Energy (USA)-funded project. The Argus II is currently the most advanced artificial retina device [31], and the only one currently implanted commercially in USA and Europe to restore partial sight to blind people (The Argus II device was named one of the top 25 inventions for 2013 by TIME magazine -November 25, 2013).
Coatings technologies for encapsulation of implantable microchips: Silicon (Si)-based microchips implanted inside the eye, should encapsulate with a hermetic/bio inert/biocompatible coating to inhibit chemical destructive attack of Si by the eye’s humour. The hermetic coatings should have the double functionality of protecting the implantable device and the surrounding tissues, to enable long service time devices, free of electronic failure. A packaging technology (titanium based hard shell) is already used in the the commercial Argus II device located outside the eye inside the shell and connected to the retina though polymer encapsulated Pt wires, to transmit charges from the microchip to the electrodes in contact with the ganglion cells on the retina). The best approach would be to have a coating encapsulating the Si-microchip, protecting it from chemical attach from the eye humor, to implant the chip fully inside the eye. The coatings should be hermetic and biocompatible, because the Si CMOS device performance can be affect by sodium ion incorporation from the eye’s saline humor, and Si can be attach chemically leading to destruction [32].
Coating materials that have been evaluate as encapsulating coatings, for Si microchips, include SiO [33], SiC [34], Polytetrafluoroethylene and polyimide [35], and parylene [36]. There are several problems with these coatings, namely: a) SiO coatings exhibited dissolution and decay after six months implantation in animals, [32] b) inexpensive flexible encapsulating polymers absorb significant quantities of water, thus, there are are not hermetic, enabling chemical attack and eventual destruction of the encapsulated chip. SiC coating performs better than the other coatings mentioned above, but they cannot be growing at temperatures ≤ 400˚C, and still there is the problem that the Si component may result in undesirable reactions.
UNCD coating technology for encapsulation of implantable microchips
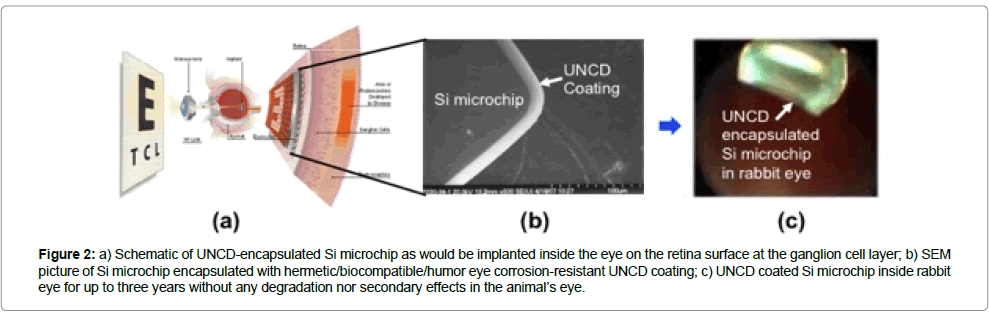
Contrary to the drawbacks of the coatings described above, UNCD coatings exhibit a unique combination of properties described above, and, for the encapsulation of artificial retina Si microchip, The UNCD coatings are the only diamond-based coatings that have been demonstrated to be grown at ≤ 400˚C (non-destructive thermal budget temperature) on CMOS devices (Figures 2 and 3a), without any degradation [1]. Extensive in vivo implantation of UNCD-coated Si chips in rabbit eyes for long periods of time (up to three years) (Figure 2c,3b), followed by scanning electron microscopy studies (Figure 3(c-e)) [30] and X-ray photoelectron spectroscopy (XPS) (Figure 3f ) [30], for surface chemical analysis, showed that the UNCD coating provides the encapsulating coating that may enable long term (years) implantation of a Si chip inside the eye without any degradation.
Figure 2: a) Schematic of UNCD-encapsulated Si microchip as would be implanted inside the eye on the retina surface at the ganglion cell layer; b) SEM picture of Si microchip encapsulated with hermetic/biocompatible/humor eye corrosion-resistant UNCD coating; c) UNCD coated Si microchip inside rabbit eye for up to three years without any degradation nor secondary effects in the animal’s eye.
Figure 3: a) Ti-based dental implant extracted from the mouth of a patient, showig the corrosion induced by the oral’s fluid; b) equal concentrations of TiO2 & UNCD particles in cell cultures yield strong programed cell death for TiO2 particles in contact with live cell, while there is practically NO cell death in contact with UNCD particles.
Details of the R&D performed to develop UNCD films as bioinert/biocompatible coatings for encapsulation of the artificial retina microchip can found [16]. On the other hand, details on the Argus II device development be found in [31,36]. The Argus II device features the microchip encapsulated in a biocompatible metallic box located outside the eye and connected, via polymer encapsulated Pt wires, to Pt electrodes on a polymer support matrix implanted on the retina’s ganglion cell layer. When approval from regulatory agencies is obtained to insert UNCD in the eye, the UNCD-encapsulated microchip could be implanted inside the eye, receiving images wireless from the CCD camera, thus, eliminating undesirable hard wire connection to the retina through the eye’s wall, as currently implemented [31,36].
Application of UNCD coatings to cover surfaces of drainage valves for treatment of glaucoma
Background: Glaucoma is the condition related to the clogging of the tubes in the trabecular mesh of the eye, which continuously evacuate the aqueous humour from the inner region of the eye towards the outside to keep the inner eye pressure constant to avoid increased intraocular pressure (IOP), which can destroy the optical nerve, leading to blindness (Figure 4) [30].
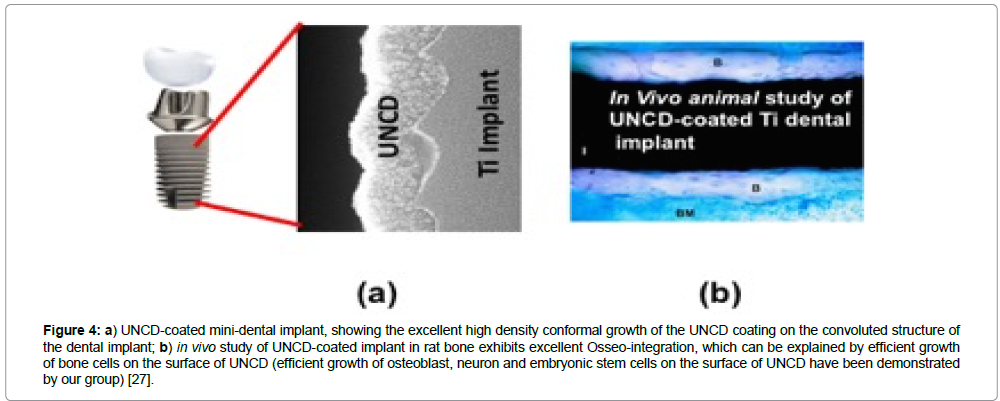
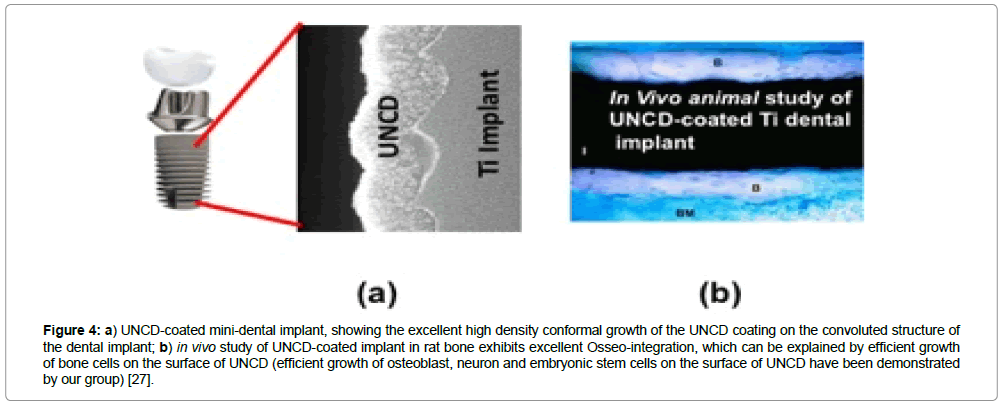
Figure 4: a) UNCD-coated mini-dental implant, showing the excellent high density conformal growth of the UNCD coating on the convoluted structure of the dental implant; b) in vivo study of UNCD-coated implant in rat bone exhibits excellent Osseo-integration, which can be explained by efficient growth of bone cells on the surface of UNCD (efficient growth of osteoblast, neuron and embryonic stem cells on the surface of UNCD have been demonstrated by our group) [27].
Other causes of glaucoma, although less common, include blunt/ chemical injury in the eye, eye infection, clogging of blood vessels or eye inflammation, and eye surgery done to correct some other eye condition. Glaucoma generally occurs in both eyes, although each is affected differently.
Glaucoma is the second leading cause of blindness after cataracts. Glaucoma affects one in 200 people aged 50 and younger, and one in 10 over the age of 80. Early detection of glaucoma arrest or slow progression, via pharmacological and surgical means. Further details about glaucoma can be found in [37].
Hydrophobic UNCD films as hermetic bio-inert/biocompatible coatings for a new generation of long life glaucoma treatment devices
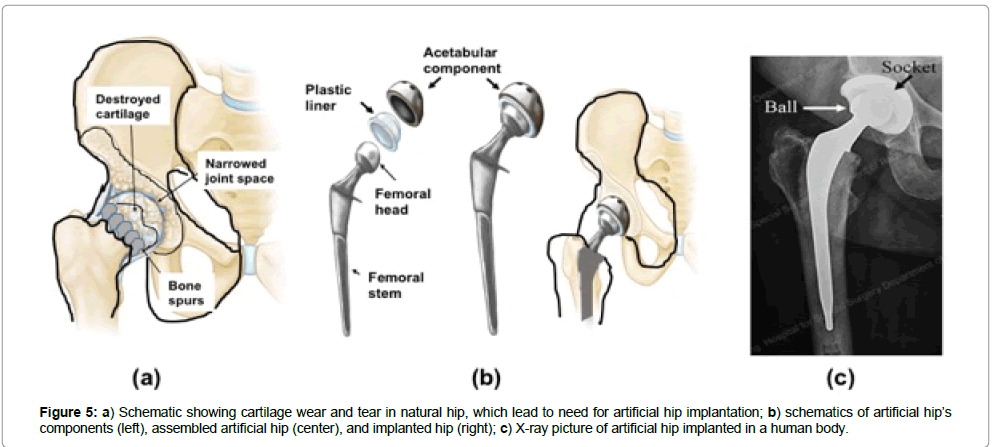
Current commercial glaucoma treatment devices inserted in the eye to drain the aqueous humour are based mainly in polymers and are rather large ( ~ 1.7 × 1 cm), and the implant surgery requires two incisions, one to insert the body of the valve in the eye sclera and the other to insert the polymer tube inside the eye to drain the fluid (Figure 5a) [30]
A problem, not yet solved, with current glaucoma treatment devices is the eye’s reaction against the implant, due to protein layer adhesion to the hydrophilic surface of the polymer, resulting in short time eye inflammation. Macrophages are attracted, by chemical signals from the eye, to the implant surface, where they release growth factors, inducing growth of fibroblast cells that yield collagen production that result in a fibrous capsule development around the implant. Subsequently, complete fibrosis occurs on the surface of the valve, isolating the implant from the rest of the body. This fibrous capsule induces materials degradation effects, not only on glaucoma valves discussed here, but also on other medical implants, resulting in degraded sensor performance or transport of drugs in drug delivery devices. Several approaches have been investigated to overcome these problems, but with not proven success.
Thus, this section focuses on presenting a brief description of animals studies done in a joint collaboration between researchers at the University of Texas-Dallas and the University of Buenos Aires and Hospital Austral in Buenos Aires, Argentina, directed at investigating the effectiveness of a UNCD coating in eliminating the development of fibrotic capsules on the surface of commercial glaucoma siliconebased valves, which over time degrade the performance of the valve to drain the eye’s humour. The animal studies were performing following NIH and ANMAT guidelines (Argentina FDA) for animal care. The studies involved two rabbits, each receiving one uncoated drainage device (Figure 5b) [30] on one eye, and one coated with a low temperature (~ 400˚C) UNCD film (Figure 5c) [30] on the other eye, in order to avoid ambiguities from using different animals in one experiment. The uncoated valve developed fibrosis, due to eye proteins adhesion, 24 hrs. after implantation, while the UNCD-coated device remained clear even after several months of implantation (Figure 5c) [30], thus demonstrating the power of UNCD films as bio-inert/biocompatible encapsulating coatings, to improve by orders of magnitude the lifetime of the implanted glaucoma valve. Approval for clinical trials are needed and our group is talking to a company manufacturing these valves to explore interest in licensing the UNCD coating technology and helping to request clinical trial approval and subsequent commercialization.
The elimination of protein adhesion on the surface of the UNCDcoated drainage device is attributed to the fact that the surface of UNCD films is extremely hydrophobic (no water adhesion), due to the hydrogen termination of the surface of the as-deposited UNCD films [1].
Application of oral fluids corrosion-resistant UNCD coatings to a new generation of dental implants with superior performance
Background: Pure Ti and Ti-alloys are widely used in dental and orthopedic implants, because past R&D indicated that Ti-based implants exhibit suitable mechanical properties and biocompatibility [37,38]. However, recent R&D on, and use of Ti-based implants in patients, indicates that they exhibit failures, specifically of Osseo integration and corrosion induce by chemical attack by body fluids [39].
Currently, worldwide statistic indicate that about 5-10% Tibased dental implants fail in shorter times than desired, due to oral fluids-induced Ti electrochemical corrosion (Figure 3a) [40]. The electrochemical corrosion process releases metal-oxide particles (mainly TiO2, since Ti oxidizes readily when exposed to air before the prostheses is implanted inside the human body) from the oxidized Ti surface into the local tissue [41], resulting in elevated levels of metal-oxide particles released from implants, which induce potential long-term systemic toxicity [42]. The corrosion of Ti surface occurs when the natural oxide passivation layer, formed by atmospheric exposure, is removed or has been partially formed only, and metallic Ti is exposed to corrosive body fluids. The metallic implant acts as an in vivo electrode leading to electrochemical degradation of the implant’s surface [40,42], weakening the implant/bone interface, thus the attachment to the bone [42]. In addition, TiO2 particles released from the implant can migrate to distant sites producing additional biological deleterious effects like killing live cells (Figure 3b) [43].
UNCD-coated metallic implants exhibit extreme resistance to chemical attack by body fluids [1,16]. In addition, roughening of the Ti implant surface by micro machining, followed by coating with UNCD films, produces a chemically resistant micro-roughened surface, which can enhance Osseo integration, as demonstrated in R&D for roughened Ti implant surfaces [44]. Carbon based materials, including diamond, have emerged as attractive coatings for biomedical implants, due to good tissue compatibility, resistance to chemical attack by body fluids, radiation resistance (suitable for sterilization processes), hemocompatibility, low friction coefficient, negligible wear, and good adhesion to Ti. In addition, recent work demonstrated that NCD coatings exhibit increased resistance to bacterial adhesion compared to stainless steel and Ti [45].
Synthesis and characterization of UNCD-coated dental implants
A Ti laminar implant was used as base material to prepare three types of implants for in vivo animal tests in the School of Dentistry, University of Buenos Aires, namely:
i) uncoated Ti,
ii) UNCD-coated Ti, and
iii) UNCD/W-coated Ti, where the W interface layer was grown by magnetron sputter-deposition to explore if it produce a denser UNCD film, as shown in prior work. The UNCD coatings were grown using the MPCVD and HFCVD methods [1].
Biological studies of dental implants with and without UNCD coating
Animal studies were performing using Male Westar rats to investigate the peri-implant reparation process. Guidelines from NIH and the Ethics regulations of the Faculty of Dentistry, University of Buenos Aires, for surgical procedures and care of laboratory animals, were observed, as done in recent work.
The histological analysis of the samples from the control and experimental groups revealed areas of lamellar bone in close contact with the surface (Osseo integration) and areas of bone marrow in contact with the implant surface (myelointegration). These studies have shown that UNCD has good biocompatibility as a coating material for dental implants. Among other factors, the clinical success of implants depends on osseointegration of the implant, and if coated, on the coating/substrate reliability. Histological studies indicated that UNCD-coated dental implants (Figure 4a) achieved excellent Osseo integration, without exhibiting any inflammatory reactions (Figure 4b) [30].
In addition, more recently, studies on chemical corrosion of UNCD-coated dental implants [47], as potentially induced by saliva, demonstrated that the surface of the UNCD coating is order of magnitude superior to bare Ti-based implants, and would resist corrosion by saliva [47].
Application of body fluids corrosion-resistant/ lowest coefficient of friction/biocompatible UNCD coatings to develop a new generation of prostheses with superior performance to current metal based prostheses
Background: Human joints are remarkable pieces of nature’s engineering, moving with negligible friction between the components, aided by healthy cartilages. The knee is the largest joint in the body. It connects the femur (thighbone), tibia (shinbone), fibula (outer shinbone), and patella (kneecap. A mixed network of muscles, ligaments, and tendons holds the knee joint together, enabling motion. The hip is another key joint in the human body, composed of two main components, i.e., a ball and a socket made of natural bone. The ball rotates inside the socket when a person walks. These key natural joints are affected by a condition known as Osteoarthritis (OA), which is the most common form of arthritis worldwide, affecting about 80% of people over 75 years old and older. In the USA, around 27 million adults currently have OA. This number is expecting to go up as life expectancy increases, with the first wave of baby boomers already reaching retirement. Typically, symptoms first begin after age 40. Through wear and tear, the cartilage wears out in certain jointsprimarily the hip (Figure 5a), knee, lower back, neck and hands — leading to stiffness, pain and eventually almost total immobility. In many cases, the natural components of the joints need to replace by artificial prostheses. The components of hip replacement prosthesis include a round ball on a stem that sits inside the thighbone and a smooth, lined socket that is attach to the pelvic bone (Figure 5b).
Ti-alloys are mainly use for fabrication of orthopedic implants (e.g., hips, knees, elbows and more), because past R&D indicated that Ti-based implants exhibit suitable mechanical properties and biocompatibility [38]. However, recent R&D on, and use of Ti-based implants in patients indicates that they exhibit failures, specifically of Osseo-integration and corrosion induced by chemical attack by body fluids [39]. One problem that was not initially recognized is that Ti and Ti-alloys oxidize readily when exposed to normal atmospheric conditions. This effect has been identified as a deleterious condition, because body fluids induce chemical attach of the TiO2 surface layer, which results in TiO2 micro (MP) and nanoparticles (NP) dislodging from the surface of the implant, with the latter, having a greater surface-to-volume ratio than MPs, and are therefore biologically more reactive and potentially more harmful to human health [48,49]. Recent histological studies of lung, liver, and kidney tissue samples showed the presence of TiO2 or UNCD particles in the parenchyma of the studied organs. It was observe that TiO2 NPs cause substantial deleterious morphological changes in the liver and kidneys, while UNCD particles caused no alterations in any of the studied organs, demonstrating the outstanding biocompatibility of UNCD [50].
Synthesis and characterization of UNCD-coated prostheses
The R&D performed on coating of industrial products like mechanical pump seals and bearing shows that UNCD coatings have great potential to solve all the problems described above in relation to failure of Ti-based prostheses (e.g., hips, knees, elbows and more), due to synergistic mechanical (high friction)/body fluid chemical attack degradation. Ti and Ti-6Al-4V (a main alloy used in fabrication of approved implantable prostheses) exhibit relatively high friction coefficients (~ 0.3 to 0.5, as measured with polytetrafluoroethylene (PTFE) and stainless steel sliding balls, respectively) [51], resulting in the wear and performance problems characteristic of current approved prostheses implanted in people today. In addition, the chemical attack by body fluids strongly contributes to the mechanicalinduced degradation, due to a synergistic combined mechanical/ chemical process.
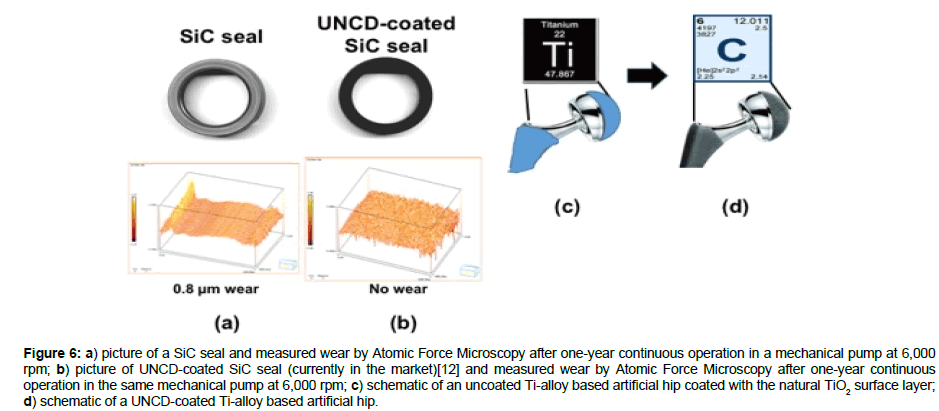
UNCD coatings are currently in commercial mechanical pumps seals and bearings, commercialized by ADT, the company co-founded by Auciello and colleagues in 2003 [12], where the UNCD coated surfaces exhibit friction coefficients in the range 0.02-0.04 (Figure 1f) [1], that is, one order of magnitude lower than the friction coefficient of any metal or ceramic used today in any approved prostheses, and practically no wear for UNCD-coated SiC mechanical pump seals (Figure 6b), while there is extensive wear for uncoated SiC seals (Figure 6a), sliding upon each other at ~ 6,000 rpm, with forces orders of magnitude higher than those present in artificial prostheses. The performance of UNCD-coated SiC seals indicates that UNCD-coated metal and ceramic prostheses used today will exhibit performance and lifetime improved by orders of magnitude over uncoated prostheses. R&D is underway to confirm and prove this hypothesis.
Figure 6: a) picture of a SiC seal and measured wear by Atomic Force Microscopy after one-year continuous operation in a mechanical pump at 6,000 rpm; b) picture of UNCD-coated SiC seal (currently in the market)[12] and measured wear by Atomic Force Microscopy after one-year continuous operation in the same mechanical pump at 6,000 rpm; c) schematic of an uncoated Ti-alloy based artificial hip coated with the natural TiO2 surface layer; d) schematic of a UNCD-coated Ti-alloy based artificial hip.
Work is currently in progress to perform animal test for UNCDcoated Ti-alloy artificial hip. An important fact is that Ti-based product is coated with excellent dense UNCD films.
Corrosion-resistant electrically conductive n-UNCD-coated electrodes and insulating UNCD-coated membranes and inner walls of cases for a new generation of LI-ion batteries for longer life implantable defibrillator/pacemaker
Lithium-ion batteries (LIBs) are in the market today powering electric vehicles and electronic devices (e.g., cell phones, laptop computers, and many other electronic systems). However, the application relevant to this article is LIBs powering of medical devices, and particularly defibrillator/pacemakers to protect people with weak heart conditions from life threatening events, due to heart failure. In spite of being in the market, LIBs still have shortcomings, and do not meet all requirements for an energy storage device for future high performance electric vehicles [52] or electronic or medical devices. LIBs require mayor improvements in electrode materials to minimize or eliminate the capacity energy degradation vs charge/ discharge cycles, due to chemical attack by Li-ions. Various anode materials exhibit excellent reversible insertion of lithium [53], via intercalation in layered carbons (e.g., graphite), adsorption in hard carbons surface layers, and binding on hydrogen atoms in carbons containing hydrogen [54]. Mesocarbon Microbeads (MCMB) are in standard anode material for LIBs due to their high theoretical capacity (372 mAh/g based on LiC6) and low potential profile (0-0.3 V Li/Li+). Natural graphite (NG) has emerged as a strong candidate anode material to replace (MCMB), because the NG high Coulombic efficiency and electronic conductivity (0.4-2.5×104 S cm-1 on the basal plane), low volume expansion after whole lithiation (~ 10%), and low cost (≤ US$10/Kg) [55]. However, large irreversible capacity loss and short charging/discharging cycling life limit the use of NG electrodes in LIBs. Chemical reactivity of Li-ion-inserted NG with organic electrolytes needs to be eliminated to produce efficient NG-based LIB anodes. Reactions of Li-ion-inserted NG with electrolytes form local solid-electrolyte-interphase (SEI) films of increasing thickness and electrical resistance, generating gases (e.g., hydrogen, CO, CO2, methane, ethylene, and propylene within interlayers of NG crystals, and resulting in irreversible capacity loss and damage to the NG structure (Figures 7b and 8), reducing battery life.
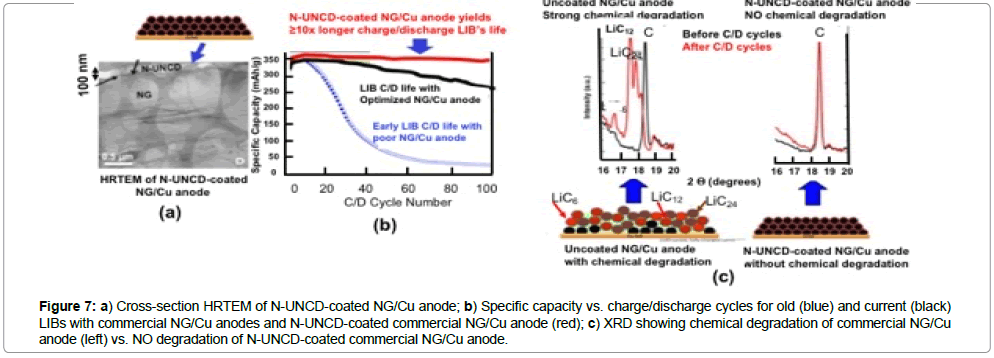
Figure 7: a) Cross-section HRTEM of N-UNCD-coated NG/Cu anode; b) Specific capacity vs. charge/discharge cycles for old (blue) and current (black) LIBs with commercial NG/Cu anodes and N-UNCD-coated commercial NG/Cu anode (red); c) XRD showing chemical degradation of commercial NG/Cu anode (left) vs. NO degradation of N-UNCD-coated commercial NG/Cu anode.
Properties of the SEI layer depend on the electrolyte compositions, microstructures and surface characteristics of the NG anode. The SEI films are formed when negative electrodes are polarized to low potentials in non-aqueous electrolytes, containing Li salts, resulting in the reduction of electrolyte species to insoluble salts. The films block electron transfer between electrodes and electrolyte, but conduct Li ion, as explained by the SEI model. However, the SEI passivation films crack due to the volume change of the graphite anode and expose fresh surface of electrodes, which is repaired by further graphite anode surface-electrolyte reaction, resulting in continuous SEI films formation and Li-ions deficiency, causing the battery capacity to fade. The process described above becomes worse on electrodes where the local charging and discharging current density is higher than average. In addition to the SEI films, dendritic growth of compound structures occurs in highly reactive graphite electrode areas, resulting in electrical shorting and hazardous failure of the LIB. The LIB anode problems described above limit increase of LIB market share for electrical vehicles, requiring inexpensive LIBs with NG anodes exhibiting long-life and high-rate charging/discharging cycle performance.
Coating of LIBs natural graphite/cu anode with electrically conductive n-UNCD and measurements of performance
Recent R&D by Auciello / Tzeng and colleagues [56,57], demonstrated that by coating the NG/Cu commercial anodes of LIBs with electrically conductive nitrogen grain boundary incorporated N-UNCD, the chemical degradation of the NG layer, due to Li-NG chemical reaction is practically eliminated (Figure 7c) and there is not degradation in the specific energy vs. charge /discharge cycles (Figure 7b-red curve).
The data shown in Figure 7 indicates that N-UNCD-coated LIBs NG/Cu anodes can enable the next generation of LIBs with ≥ 10x longer life and safer to enable the next generation of defibrillator/ pacemaker with orders of magnitude longer life than current devices, which may not require change in many more years than current LIBs require. Further R&D is underway also develop N-UNCD-based coatings for cathodes and insulating UNCD coatings for membranes and inner walls of LIBs’ cases.
Summary view of the multiple applications of the biocompatible UNCD coating technology
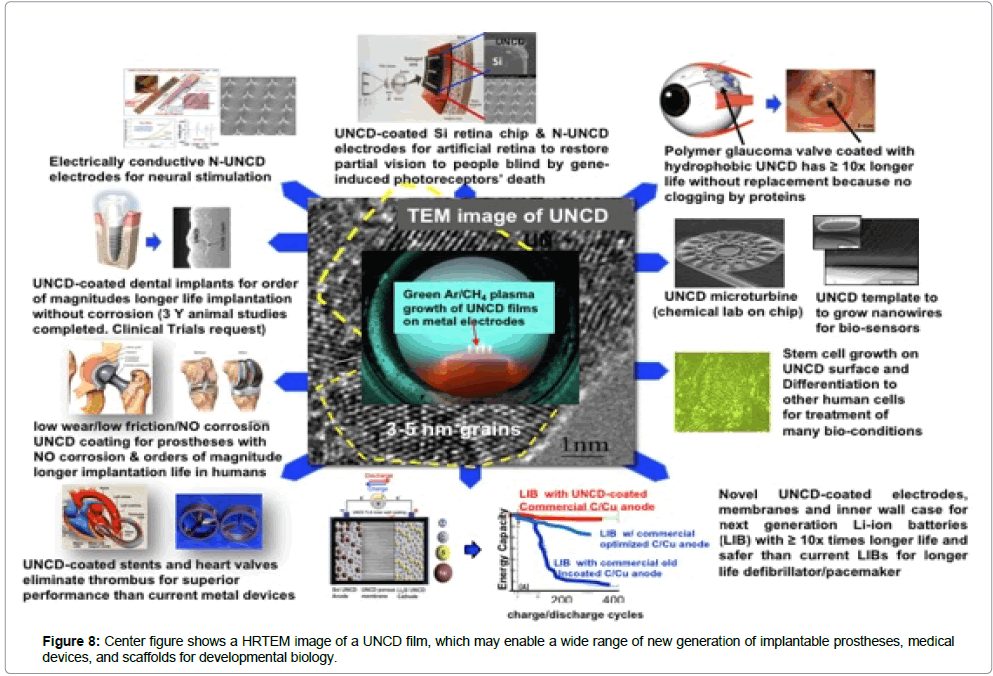
A summary of the multiple applications of the novel UNCD coating, to enable the next generation of implantable prostheses and other medical devices, and a new generation of scaffolds with superior surface chemistry for stem cell growth and differentiation for developmental biology.
Summary
The data from R&D projects discussed in this review, on the fundamental and applied materials science and implant and device development and testing for some key medical implant and devices based on UNCD coatings shows that this material provides a unique combination of mechanical, tribological, electrical, chemical and biological properties, which may enable a new generation of medical implants and devices with superior performance with respect to those currently based on materials currently used in commercial devices and implants, which exhibit serious limitations n performance.
In addition, the work described in this article also shows that UNCD coatings provide superior scaffolds for stem cell growth and differentiation. Work is in progress to develop and introduce all products into the market.
Acknowledgements
Work performed at the University of Texas-Dallas was supported by the Endowed Chair funds of Prof. O. Auciello (#37139010). Work related to the artificial retina project was funded by the DOE-Artificial Retina Project, and Auciello acknowledges the outstanding collaboration with all team members, and especially Dr. M. Humayun (Director of the artificial retina project), and P. Gurman for contributions to the work on UNCD coating of the Si-microchip. Auciello acknowledges the strong contributions of Drs. M. Saravia (Hospital Austral-Argentina), P. Gurman (UTD), and A. Berra (Universidad de Buenos Aires-Argentina) on the R&D related to the development of UNCD-coated glaucoma valves. Auciello acknowledges the outstanding contributions of Profs. M. Guglielmotti, D. Olmedo, D. Tasat. M. Bruno, M. Domingo, M. Paparella, and P. Evelson (University of Buenos Aires (UBA)-Argentina), and P. Gurman and K. Kang (UTD) for the work on UNCD-coated dental implants (work in UBA-Argentina was funded by grants: UBACyT 20020100100812 (UBA); PIP 11220090100117 (CONICET) and PDTS PO 01 2013 (UBA)-Ministry of Science, Technology and Productive Innovation, Argentina. Auciello acknowledges the outstanding contributions of Prof. T. Tzeng, Y-W. Cheng, C-K. Lin, and Y.-C. Chu (NCKU-Taiwan), A. Abouimrane, Z. Chen,Y. Ren, and C.-P. Liu (Argonne National Laboratory-USA), for the work on N-UNCD-coated anodes for LIBs.
References
- Auciello O, Sumant AV (2010) Status review of the science and technology of Ultrananocrystalline diamond (UNCDTM) films and application to multifunctional devices. Diam Relat Mater 19: 699-718.
- Gruen DG, Krauss AR, Auciello O, Carlisle JA (2004) N-Type doping of NCD films with nitrogen and electrodes made there from. US patent #6,793,849 B1.
- Naguib N, Birrell J, Elam J, Carlisle JA, Auciello O (2006) A method to grow carbon thin films consisting entirely of diamond grains 3-5 nm in size and high-energy grain boundaries. US Patent #7,128,8893.
- Carlisle JA, Gruen DM, Auciello O, Xiao X (2002) A method to grow pure nan crystalline diamond films at low temperatures and high deposition rates. US Patent # 7,556,982.
- Tzeng Y, Auciello O, Liu CP, Lin CK, Cheng YW,et al.(2015) Nanocrystalline-diamond/ carbon and nanocrystalline-diamond/silicon composite electrodes for Li-based batteries. US Patent # 9,196,905.
- Butler JE, Sumant AV (2008) The CVD of nanodiamond materials. Chem Vap Deposition 14: 145-160.
- Alcantar-Peña JJ, Yacaman MJ, Auciello O, Arellano-Jimenez MJ, Berman-Mendoza D, et al. (2016) Low temperature hot filament chemical vapor deposition of Ultrananocrystalline Diamond films with tunable sheet resistance for electronic power devices. Diam Relat Mater 69: 207-213.
- Fuentes-Fernandez EMA, Alcantar-Peña JJ, Lee G, Boulom A, Phan H, et al. (2016) Synthesis and characterization of microcrystalline diamond to ultrananocrystalline diamond films via hot filament chemical vapor deposition for scaling to large area applications. Thin Solid Films 603: 62-68.
- Hao T, Zhang H, Shi C, Han G (2006) Nano-crystalline diamond films synthesized at low temperature and low pressure by hot filament chemical vapor deposition. Surf Coat Tech 201: 801-806.
- May PW, Smith JA, Mankelevich YA (2006) Deposition of NCD films using hot filament CVD and Ar/CH4/H2 gas mixtures. Diam Relat Mater 15: 345-352.
- Schwarz S, Rosiwal SM, Frank M, Breidt D, Singer RF (2002) Dependence of the growth rate, quality, and morphology of diamond coatings on the pressure during the CVD-process in an industrial hot filament plant. Diam Relat Mater 11: 589-595.
- Advanced Diamond Technologies, Commercial Production of UNCD Coating-Based Industrial Products via HFCVD process, www.thindiamond.com, 2003 - present.
- Sternberg M, Zapol P, Curtiss LA (2003) Carbon dimers on the diamond (100) surface: growth and nucleation. Phys Rev 68 :205330.
- May PW, Allan NL, Ashfold MNR, Richley JC, Mankelevich YA (2009) Simplified Monte Carlo simulations of chemical vapor deposition diamond growth J Phys Condens Matter 21: 364203.
- Xiao X, Birrell J, Gerbi JE, Auciello O, Carlisle JA (2004) Low temperature growth of Ultrananocrystalline Diamond. J Appl Phys 96: 2232-2239.
- XiaoX,Wang J, Carlisle JA, Mech B, Auciello O et al. (2006) In Vitro and In Vivo Evaluation of Ultrananocrystalline Diamond for coating of implantable retinal microchips. J Biomedical Materials 77: 273-281.
- May PW, Smith JA, Mankelevich YA (2006) Deposition of NCD films using hot filament CVD and Ar/CH4/H2 gas mixtures. Diam Relat Mater 15: 345-352.
- Schwarz S, Rosiwal SM, Frank M, Breidt D, Singer RF (2002) Dependence of the growth rate, quality, and morphology of diamond coatings on the pressure during the CVD-process in an industrial hot filament plant. Diam Relat Mater 11: 589-595.
- Sternberg M, Zapol P, Curtiss LA (2003) Carbon dimers on the diamond (100) surface: growth and nucleation. Phys Rev 68 :205330.
- May PW, Allan NL, Ashfold MNR, Richley JC, Mankelevich YA (2009) Simplified Monte Carlo simulations of chemical vapor deposition diamond growth J Phys Condens Matter 21: 364203.
- Xiao X, Birrell J, Gerbi JE, Auciello O, Carlisle JA (2004) Low temperature growth of Ultrananocrystalline Diamond. J Appl Phys 96: 2232-2239.
- Sumant AV, Auciello O, Carpick RW, Srinivasan S, Butler JE (2010) Ultrananocrystalline and Nanocrystalline Diamond thin films for MEMS/NEMS applications. MRS Bulletin 35: 1-8.
- Yugo S, Kanai T, Kimura T, Muto T (1991) Generation of diamond nuclei by electric field in plasma chemical vapor deposition. Appl Phys Lett 58: 1036-1038.
- Janischowsky K, Ebert W, Kohn E (2003) Bias enhanced nucleation of diamond on silicon (100) in a HFCVD system. Diam Relat Mater 12: 336-339.
- Ansari SG, Anh TL, Seo KH, Sung KG, Mushtaq D, et al. (2004) Growth kinetics of diamond film with bias enhanced nucleation and H2/CH4/Ar mixture in a hot-filament chemical vapor deposition system. J Cryst Growth 265: 563-566.
- Alcantar-Peña JJ, de Obaldia, Montes-Gutierrez JE, Kang Auciello O, et al. (2017) Fundamentals towards large area synthesis of multifunctional Ultrananocrystalline Diamond Films via large area hot filament chemical vapor deposition bias enhanced nucleation/bias enhanced growth for fabrication of broad range of multifunctional devices. Diam Relat Mater 78: 1-11.
- Lee YC, Lin SJ, Chia CT, Cheng HF, Lin IN (2005) Effect of processing parameters on the nucleation behavior of nano-crystalline diamond film. Diam Relat Mater 14: 296-301.
- House WF (1976) Cochlear implants. Ann Otol Rhinol Laryngol 85: 1-93.
- Weiland JD, Liu W, Humayun MS (2005) Retinal prosthesis. Annu Rev Biomed Eng 7: 361-401.
- Chen YC, Zhong XY, Konicek AR, Grierson DS, Auciello O, et al. (2008) Synthesis and characterization of smooth ultrananocrystalline diamond films via low pressure bias-enhanced nucleation and growth. Appl Phys Lett 92: 133113.
- Stoner BR, Ma G-H, Wolter SD, Glass JT (1992) Characterization of bias-enhanced nucleation of diamond on silicon by invacuo surface analysis and transmission electron microscopy. Phys Rev B 45: 11067-11084.
- Garrett DJ, Ganesan K, Stacey A, Fox K, Meffin KH, et al. (2012). Ultrananocrystalline diamond electrodes: optimization towards neural stimulation applications. J Neural Eng 9: 016002.
- Meyer J (2001) Retina Implant- bioMEMS challenge. Sensor and Actuator A 97-98: 1-9.
- Zhanga J, Zimmer JW, Howe RT, Maboudian (2008) Characterization of boron-doped micro- and nanocrystalline diamond films deposited by wafer-scale hot filament chemical vapor deposition for MEMS applications. Diam Relat Mater 17: 23-28.
- Shi B, Jin Q, Chen L, Auciello O (2009) Fundamentals of Ultrananocrystalline Diamond (UNCD) thin films as biomaterials for developmental biology: embryonic fibroblasts growth on the surface of (UNCD) films. Diam Relat Mater 18: 596-600.
- Auciello O, Gurman P, Guglielmotti MB, Olmedo DG, Berra A, et al. (2014) Biocompatible Ultrananocrystalline Diamond Coatings for Implantable Medical Devices. MRS Bulletin, 39: 621-629.
- Humayun M (2012) Interim results from the international trial of second sight's visual prosthesis. Ophthalmol 199: 779-788.
- Hammerle H, Kobuch K, Kohler K, Nisch W, Sachs H, et al. (2002) Biostability of micro-photodiode arrays for subretinal implantation. Biomater 23: 797-804.
- Cogan SF, Edell D, Guzelian A, Liu Y, Edell R (2003) Plasma-enhanced chemical vapor deposited silicon carbide as an implantable dielectric coating. J Biomedical Mater Res 67: 856-867.
- Seo J, Kim S, Chung H, Kim H, Yu H, et al. (2004) Biocompatibility of polyimide microelectrode array for retinal stimulation. Mater Sci Engin C 24: 185-189.
- Zhou DD, Greenbaum Eds (2010) Implantable Neural Prostheses. Springer 1&2.
- Auciello O, Gurman P, Berra A, Zaravia M, Zysler R (2013) Diamond based materials for biomedical applications. Roger Narayan (Ed.), Woodhead Publishing 151.
- Abdel-Hady Gepreel M, Niinomi M (2013) Biocompatibility of Ti-alloys for long-term implantation. J Mech Behav Biomed Mater 20: 407-415.
- Palmquist A, Omar OM, Esposito M, Lausmaa J, Thomsen P (2010) Titanium oral implants: surface characteristics, interface biology and clinical outcome. J R Soc Interface 7: S515-S527.
- Gittens RA, Olivares-Navarrete R, Tannenbaum R, Boyan BD, Schwartz Z (2011) Electrical implications of corrosion for osseointegration of titanium implants. J Dent Res 90: 1389-1397.
- Olmedo DG, Cabrini RL, Duffó G, Guglielmotti MB (2008) Local effect of titanium implant corrosion: an experimental study in rats. Int J Oral Maxillofac Surg 37: 1032-1038.
- Olmedo DG, Tasat DR, Evelson P, Rebagliatti R, Guglielmotti MB, et al. (2011) In vivo comparative biokinetics and biocompatibility of titanium and zirconium micro particles. J Biomedical Mater Res 98: 604-613.
- Bruno ME, Tasat DR, Ramos E, Paparella ML, Evelson, et al. (2014) Histo-pathological parameters: Impact through time of different sized titanium dioxide particles. J Biomed Mater Res A102: 1439-1449.
- Olmedo DG, Paparella ML, Spielberg M, Brandizzi D, Guglielmotti MB, et al. (2012) Oral mucosa tissue response to titanium cover screws. J Periodontol 83: 973-980.
- Hacking SA, Boyraz P, Powers BM, Sen-Gupta E, Kucharski W, et al. (2012) Surface roughness enhances the osseointegration of titanium headposts in non-human primates. J Neurosci Methods 211: 237-244.
- Amaral M, Gomes PS, Lopes MA, Santos JD, Silva RF (2008) Nanocrystalline Diamond as a coating for joint implants: Cytotoxicity and Biocompatibility Assessment. J Nanomater 894352.
- Shi B, Jin Q, Chen L, Auciello O, (2008) Fundamentals of Ultrananocrystalline Diamond (UNCD) Thin Films as Biomaterials for Developmental Biology: Embryonic Fibroblasts Growth on the Surface of (UNCD) Films.Diamond and Related Materials 18: 596- 600.
- Patel B, Duran-Martinez AC, Gurman P, Auciello O, Barao V, et al. (2017) Ultrananocrystalline diamond coatings for the dental implant: electrochemical nature. Surface Innovations 5: 106-118.
- Oberdörster G, Oberdörster E, Oberdörster J (2005) An emerging discipline evolving from studies of ultrafine particles. J Nanotoxicology 113: 823-839.
- Tasat DR, Bruno ME, Domingo M, Gurman P, Auciello O, et al. (2016) Biokinetics and tissue response to Ultrananocrystalline diamond nanoparticles for biomedical devices. J Biomed Mater Res Part B: Appl Mater 008.
- Tzeng Y, Auciello O, Liu CP, Lin CK, Cheng YW (2015) Nano crystalline-diamond/carbon and Nano crystalline diamond/silicon composite electrodes for li-based batteries. US Patent # 9,196,905.
- Cheng YW, Lin CK, Chu YC, Tzeng Y, Auciello O, et al. (2014) Electrically Conductive Ultrananocrystalline Diamond-Coated Natural Graphite-Copper Anode for New Long Life Lithium-Ion Battery Advanced Materials 26: 3724-3729.