Research Article, Androl Gynecol Curr Res Vol: 10 Issue: 3
Tobacco and Alcohol Consumption Impairment of Sperm Morphology: its Relationship with Seminal Oxidative Stress and Socio–Demographic Characteristics
Oumaima Ammar1,2*, Mohamed Habib Aoun3, Tesnim Ajina1,Mouna Gara4, Ali Jlali4 and Meriem Mehdi1,2
1Department of Histology Embryology and Cytogenetics, University of Monastir, Monastir, Tunisia
2Department of Cytogenetics and Reproductive Biology, Monastir, Tunisia
3Department of Psychiatry, Tahar Sfar University Teaching Hospital, Mahdia, Tunisia
4Department of Anaesthesia, Center of Maternity and Neonatology, Monastir, Fattouma Bourguiba University Teaching Hospital, Monastir, Tunisia
*Corresponding Author :Oumaima AmmarDepartment of Histology Embryology and Cytogenetics, University of Monastir, Monastir, Tunisia; E-mail: ammaroumayma2014@gmail.com
Received: 09 November, 2021, Manuscript No. AGCR-21-47079;
Editor assigned: 11 November, 2021, PreQC No. AGCR-21-47079 (PQ);
Reviewed: 26 November, 2021,QC No. AGCR-21-47079;
Revised: 10 January, 2022,Manuscript No. AGCR-21-47079 (R);
Published: 01 April 2022, DOI: 10.4172/2327-4360.1000131.
Citation: Oumaima A, Mohamed HA, Tesnim A, Mouna G, Ali J, Meriem M (2022) Tobacco and Alcohol ConsumptionImpairment of Sperm Morphology: its Relationship with Seminal Oxidative Stress and Socio-Demographic Characteristics. Androl Gynecol: Curr Res 10:3.
Abstract
Teratozoospermia is one of the most important factors contributing to male infertility. To provide some evidence for its pathogenesis, we aimed to investigate the link between smoking and alcohol consumption, oxidative stress markers, and morphological spermatic characteristics in infertile men with isolated teratozoospermia. The semen samples were obtained from 60 male partners with isolated teratozoospermia. These subjects were categorized based on self-reported history of smoking and alcohol consumption: (non-smokers, n=31; smokers, n=29) and (non-drinkers, n=32; drinkers, n=28). The ability of spermatozoa to produce superoxide anion (O2.) was assessed with the Nitro Blue Tetrazolium (NBT) staining test. Seminal antioxidant enzymes activities including Superoxide Dismutase (SOD), Catalase (CAT) Glutathione Peroxidase (GPx) were measured spectrophotometrically. The conventional sperm parameters and the detailed morphological characteristics were found to be significantly different in the different study groups. Seminal leukocytes concentration, irregular head, microcephalic head, double head, mid-piece abnormalities, and the presence of cytoplasmic excess were significantly higher in smoking patients with teratozoospermia when compared to non-smokers teratozoospermic patients (p<0.05). On the other hand, alcoholic teratozoospermic patients showed significantly higher rates of irregular head, macrocephalic head, microcephalic head, mid-piece abnormalities, and the presence of cytoplasmic excess when compared to non-drinkers (p<0.05). Besides, the levels of the studied antioxidant enzymes were reduced and sperm ROS production was higher among alcohol/tobacco consumers with teratozoospermia, though the changes were not significant (p>0.05). These findings indicated that tobacco and alcohol abuse might act as lifestyle risk factors for the increase in morphological abnormalities of spermatozoa and seminal oxidative stress damage.
Keywords: Teratozoospermia; Male infertility; Antioxidant enzymes; ROS overproduction; Tobacco smoking; alcohol abuse
Introduction
Teratozoospermia is a condition characterized by a large number of spermatozoa with abnormal morphology may result in male infertility. Over the last decade, both molecular and biochemical mechanisms underlying teratozoospermia have been of great interest for researchers [1].
We have previously shown that sperm DNA defects as well as programmed sperm cell death and Oxidative Stress (OS) can be interlinked in the context of teratozoospermia, and may constitute a unified pathogenic molecular mechanism. Superoxide anion (O2) produced from morphologically abnormal spermatozoa as well as reduced seminal antioxidant defences and protamination, induces many changes in sperm cells; including membrane and DNA fragmentation and apoptosis, which then disturbs spermatozoon production and maturation. Besides, the etiology of teratozoospermia is under investigation with a variety of factors contributing to its pathogenesis. This has drawn attention to study the impact of lifestyle and environmental factors, especially smoking and alcohol intake, on the reproductive status of teratozoospermic men [2].
Alcohol and tobacco consumption are recognized as the most modifiable risk factors that contribute to male infertility. Tobacco consumption is globally on the rise and approximately 37% of men in reproductive age are smokers [3].
Smoking has detrimental effects on semen quality, including semen volume, density, motility, viability, and normal morphology. Smoking also triggers a chronic inflammatory response which recruits leukocytes to the genital tract and causes a substantial increase in seminal Reactive Oxygen Species (ROS) levels, as well as an increase in sperm DNA damage, aneuploidies, mutations, even in apoptotic spermatogenic cells. Seminal plasma abnormalities, including impaired spermatogenesis, atypical sperm morphology, and low seminal volume, decreased levels of testosterone, and increased OS, have been associated with excessive alcohol consumption. The condition is further aggravated when tobacco use and alcohol intake co-exist.
Admittedly, there is public and scientific concern about the potential reproductive health effects of both tobacco and alcohol consumption, but little is known about the link between smoking and alcoholism, OS markers, and teratozoospermia. Therefore, by comparing semen morphological characteristics and OS biomarkers between drinkers /smokers and non-drinkers /non-smokers, we aimed to investigate the impact of tobacco and alcohol consumption on semen quality of infertile men with isolated teratozoospermia and its relationship with seminal OS [4].
Materials and Methods
Study populationThis is a controlled, descriptive, and analytical study including 60 patients who visited the Laboratory of Cytogenetics and Reproductive Biology at the Center of Maternity and Neonatal care for fertility problems, (Monastir, Tunisia). The selected patient group has an isolated polymorphic teratozoospermia in spermiogram. The patient’s occupation, alcohol consumption and smoking habits were detailed. All patients do not have a history of chemotherapy, radiotherapy or chronic illness. The study was approved by the local Ethics Committee and all patients gave their consent to participate in the study (verbal and written) [5].
Clinical characterizationThis involves the socio-demographic characteristics of the patient and his history. These clinical characteristics were collected using patients’ information based on their socio-demographic characteristics (age and occupation) and their Lifestyle (smoking and alcohol consumption) [6].
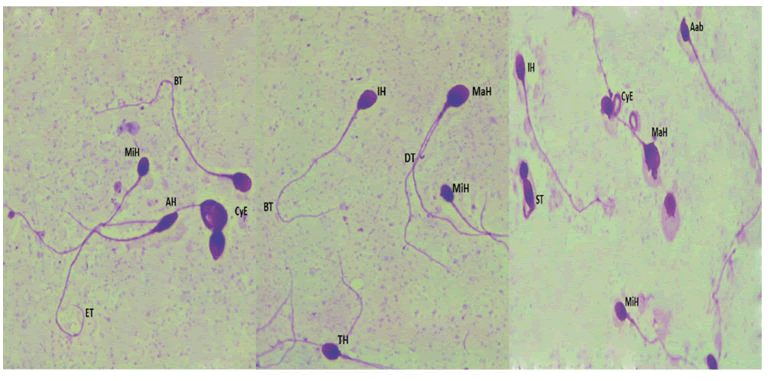
Collection, analysis and preparation of sperm samplesAll semen samples were obtained by masturbation after 3 days of sexual abstinence and collected into sterile containers. After liquefaction, standard semen parameters were evaluated according to the World Health Organization guidelines. Sperm morphology was evaluated using the Eukitt kit (Sigma Aldrich; French). At least 100 spermatozoa per patient were examined at a magnificent of X 100 according to the modified David classification Figure 1[7].
MiH: Microcephalic Head, MaH: Macrocephalic Head, AH: Aminced Head, TH: Tapred Head, IH: Irregular Head, Aab: Acrosomal abnormalities, DT: Double Tail, ST: Short Tail, BT: Bent tail, ET: Enrouled tail, CyE: Cytoplasmic Excess.
After semen analysis, each fresh semen sample was washed twice in phosphate-buffered saline (PBS, pH 7.4) and centrifuged at 3,500 RPM for 10 min to obtain seminal plasma and sperm pellet. Seminal plasma was frozen at -20°C until antioxidant enzymes activities assessment. The sperm pellet was washed twice in PBS (pH 7.4) and centrifuged at 2000 RPM for 5 min. The sediment was then fixed in methanol / acetic acid (3:1) for at least 30 min at 4°C and stored at -20°C until the Nitro Blue Tetrazolium (NBT) test [8].
Assessment of oxidative stress in semen samplesCatalase activity: Seminal plasma catalase (CAT) activity was measured according to the protocol published by Clairbone (25). Briefly, 20 μl of the seminal plasma was added in the quartz corvette containing 780 μl of phosphate buffer and 200 μl of H2O2, 0.5 M. The enzyme activity was measured by determining the maximum absorbance at 240 nm using a [9] molar extinction coefficient of 0.04/mM/cm. One unit of activity is equal to the nmol of strongO2 consumed/min/mg protein [10].
Glutathione peroxidase activityThe assessment of Glutathione Peroxidase Activity (GPx) in seminal plasma was performed according to the protocol established by Flohe and Gunzler method. The activity was expressed as μmol of GSH oxidized/min/mg of protein, at 25°C.
Superoxide dismutase activityThe measurement of Superoxide Dismutase (SOD) activity was carried out according to the Beyer and Fridovich method (27). SOD activity was evaluated by its ability to reduce NBT oxidation. The optical density was measured at 580 nm. One unit of SOD is the number of enzymes required to inhibit NBT oxidation at 50%. This activity was expressed in U/mg protein [12].
Sperm ROS productionA modified colorimetric Nitro Blue Tetrazolium (NBT) staining test was used to evaluate reactive oxygen species production of sperm cells within the semen. After PBS washing, sperm was resuspended in 200 μl of PBS and incubated with NBT reagent (0.01% NBT in PBS; Sigma-Aldrich, St. Louis, USA) for 45 min at 37°C. Following incubation, the samples were washed and centrifuged at 1000 rpm for 10 min. the sperm pellet was then smeared on slides. The slides were stained with Giemsa for 20 min and observed under an optical microscope. On each slide, a total of 100 cells were counted [13].
Statistical analysisThe statistical analysis was conducted using the statistical software: Statistical Package for Social Sciences 21.0 version. For the qualitative variables, we determined the relative and absolute frequencies. The quantitative variables were determined using means and standard deviations (Means ± SD) when the distribution was Gaussian and the median with the minimum and maximum when the distribution was non-Gaussian.
We used the Chi square and Fisher test to compare the two frequencies. When the rules for applying the Chi square test were not possible, we used the Fisher test. For the comparison of two means, we used the Student T-test for independent samples when the distribution was Gaussian and the Mann Whitney U-test when the distribution was non-Gaussian. The correlation between variables was calculated using Spearman’s non-parametric methods. In addition, multivariable regression analysis was performed to determine the life style factor that could be independently associated with OS biomarkers [14].
Results
Descriptive analysis of the study populationThe 60 recruited patients who consulted the Laboratory of Cytogenetics and Reproductive Biology of the Maternity and Neonatal Center of Monastir responded to the inclusion criteria for our study population. The mean age of patients was 33.03 ± 5.18 years with extremes ranging from 20 to 47 years, The 31-40 years age group was the most represented. In this work, we tried to study all the factors that could potentially influence the spermatic parameters of the different subjects of the population, such as [15]
Occupational exposure: Occupations were very diverse within our patient group. This group was divided into two [16]
Specific categories
- Occupations exposed to heat include bakers, builders, merchants (with prolonged sitting position), policemen (with prolonged standing position), fishermen (exposed to the sun), and drivers (truck or taxi drivers).
- Occupations exposed to chemicals, whiteners, pesticides and insecticides include painters and farmers [17].
In our study population, 56.7% of patients (34/60) were not exposed toheat and chemical products, 38.3% (23/60) were exposed just to heat and only 5% (3/60) were exposed to chemicals.
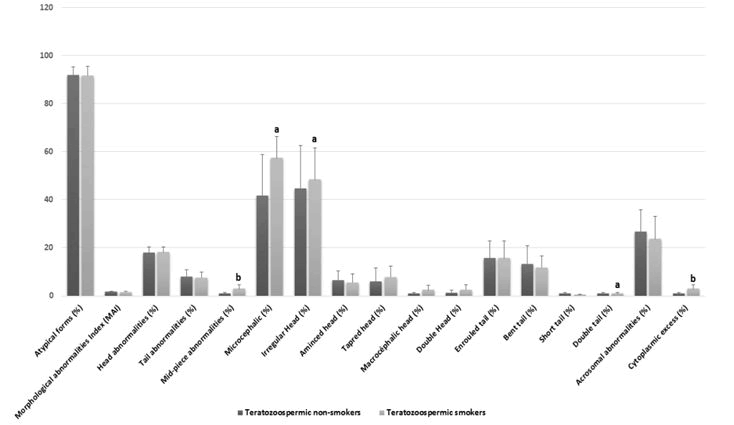
Comparison of standard spermatic parameters, detailed sperm morphological characteristics, oxidative stress biomarkers among non-smokers teratozoospermic and teratozoospermic smokers.Smoking habit was observed among the 48.33% (n=29/60) of teratozoospermic patients. Standard sperm parameters were different in samples from teratozoospermic non-smokers and teratozoospermic smokers, but this difference was only significant with the concentration of leukocytes (p=0.028) Table 1. On the other hand, [18] we noted that some specific morphological abnormalities Figure 1 were statistically higher in the semen of smoking patients as compared to non-smokers with a significant difference, for different types of head abnormalities such the irregular head (p=0.02), microcephalic head (p=0.04), and double head (p=0.02). The difference was also significant in other specific morphological abnormalities such asmid-piece abnormalities (p=0.006), and the presence of cytoplasmic excess (p=0.006), Figure 2 and Table 1.
| Teratozoospermic non-smokers (N=31; 51.66%) |
Teratozoospermic smokers (N=29; 48.33%) |
P-value | |
|---|---|---|---|
| Age | 32.53 ± 6.067 | 33.6 ± 4.16 | 0.43 |
| Volume | 3.73 ± 1.47 | 4.33 ± 2.3 | 0.26 |
| pH | 8.03 ± 0.24 | 8.037 ± 0.24 | 0.97 |
| Concentration (106 spz/ml) | 94.36 ± 0.56 | 102.4 ± 880.8 | 0.69 |
| Total Motility (%) | 48.33 ± 5.95 | 47.08 ± 6.202 | 0.52 |
| Leukocytes Concentration (106/ml) | 0.4 ± 0.38 | 0.68 ± 0.36 | 0.028 a |
| Necrozoospermia (%) | 15.94 ± 7.06 | 16.7±9.04 | 0.73 |
A Significant difference between groups (p < 0.05);
All values are expressed as mean ± standard deviation and analysed using the paired t test.
Table 1: Comparison of age and sperm parameters among Teratozoospermic smokers and Teratozoospermic non-smokers
For the studied oxidative stress biomarkers, we noted that ROS production was higher in smokers as compared to non-smokers, but this difference was not significant (p=0.58). In addition, the differences among the GPX, SOD, and CAT activities of were not statistically significant between the smoking and non-smoking patients (p>0.05), [19] Table 2.
| Teratozoospermic non-smokers (N=31; 51.66%) |
Teratozoospermic smokers (N=29; 48.33%) |
P-Value | |
|---|---|---|---|
| Oxidative stress biomarkers | |||
| ROS production (%) | 70.45 ± 10.19 | 71.78 ± 8.56 | 0.58 |
| SOD (U/mg P) | 46.55 ± 8.23 | 47.37 ± 7.27 | 0.68 |
| GPX (mmol oxide GSH/min/mg P) | 231.40 ± 212.64 | 165.67 ± 96.78 | 0.13 |
| CAT (mmol H2O2/min/mg P) | 2561.91 ± 1410.02 | 2798.79 ± 1321.80 | 0.5 |
All values are expressed as mean ± standard deviation and analysed using the paired t test. Significant difference between groups (p<0.05); b significant difference between groups (p<0.01)
Table 2: Comparison of oxidative stress biomarkers among Teratozoospermic non-smokers and Teratozoospermic smokers
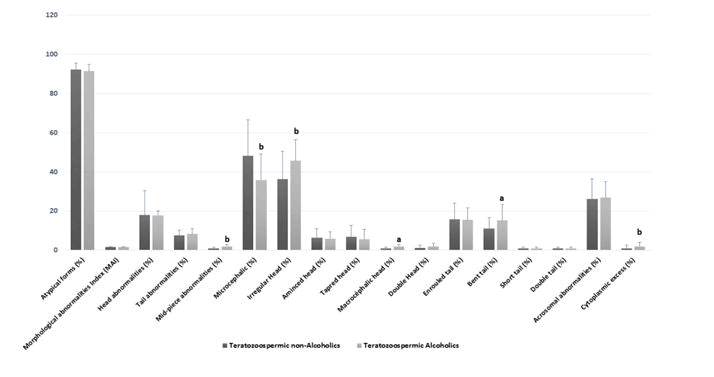
Comparison of standard spermatic parameters, detailed sperm morphological characteristics, oxidative stress biomarkers among teratozoospermic alcoholics and teratozoospermic non-alcoholics.Alcohol consumption was observed in 46.67% (N=28/60) of the teratozoospermic group. Table 3 shows that the mean age, volume, pH, concentration, total motility, leukocytes, and necrozoospermia were not significantly different among the [20] teratozoospermic alcoholics and teratozoospermic non-alcoholics (p>0.05). In contrast, some head morphological abnormalities were significantly higher in the spermocytogram of patients who drink alcohol when compared to those woes do not. This was the case of the irregular head (p=0.006), macrocephalic head (p=0.02) and microcephalic head (p=0.007) and other specific morphological abnormalities such as mid-piece abnormalities (p=0.01), and the presence of cytoplasmic excess (p=0.01) [21] Figure 3 and Table 3.
|
Teratozoospermic non-Alcoholics (N=32; 53.33%) | Teratozoospermic Alcoholics (N=28; 46.67%) | P-value |
|---|---|---|---|
| Age | 32.72 ± 5.604 | 33.46 ± 4.29 | 0.58 |
| Volume | 3.64 ± 1.41 | 4.1 ± 1.91 | 0.28 |
| pH | 8.01 ± 0.25 | 8.06 ± 0.21 | 0.43 |
| Concentration (106 spz/ml) | 92.37 ± 55.44 | 100.08 ± 71.28 | 0.64 |
| Total Motility (%) | 48.91 ± 6.05 | 47.14 ± .84 | 0.25 |
| Leukocytes Concentration (106/ml) | 0.46 ± 0.45 | 0.46 ± 0.33 | 0.96 |
| Necrozoospermia (%) | 15.5 ± 6.91 | 16.79 ± 80.29 | 0.5 |
All values are expressed as mean ± standard deviation and analysed using the paired t test
Table 3: Comparison of age and sperm parameters among Teratozoospermic non-Alcoholics and Teratozoospermic Alcoholics
ROS production was higher in the drinking group as compared to the non-drinking one, but this difference was not significant (p=0.57). Moreover, GPX and catalase activities were higher in the non-drinking group as compared to drinking [22] patients, but these differences were not significant (p=0.24 and p=0.25, respectively), while SOD activity was equal in both groups (p=0.99) Table 4.
| Teratozoospermic non-Alcoholics (N=32; 53.33%) | Teratozoospermic Alcoholics (N=28; 46.67%) | P-Value | |
|---|---|---|---|
| Oxidative stress biomarkers | |||
| ROS production (%) | 70.73±10.01 | 72.39±6.01 | 0.57 |
| SOD | 46.94±8.14 | 46.98±6.26 | 0.99 |
| GPX | 213.11±184.29 | 150.87±82.52 | 0.24 |
| CAT | 2781.91±1405.87 | 2295.96±1157.68 | 0.25 |
All values are expressed as mean ± standard deviation and analysed using the paired t test. a significant difference between groups (p < 0.05)
Table 4: Comparison oxidative stress biomarkers among Teratozoospermic non-alcoholics and Teratozoospermic alcoholics.
Correlations between the seminal OS biomarkers and sperm morphological characteristicsIn this study we investigated the correlations between seminal OS biomarkers and sperm morphological characteristics among tobacco and alcohol users (Table 5). In both groups of teratozoospermic smokers and teratozoospermic alcoholics, significant correlations were respectively found between the increased amount of NBT with the level of atypical sperm forms (r=0.406, p<0.01; r=0.409, p<0.01) and the multiple anomalies index (r=0.259, p<0.05; r=0.253, p=0.05). Moreover, the catalase activity was also [23] negatively correlated with the level of atypical sperm forms (p<0.05).
| NBT | SOD | Gpx | Catalase | |||
|---|---|---|---|---|---|---|
| Atypical forms (%) | Tobacoo | r=0.406 p<0.01 | r=-0.317 p<0.05 | |||
| Alcohol | r= 0.409 p<0.01 | NS | NS | r= -0.321 p<0.05 | ||
| Head abnormalities (%) | Tobacoo | NS | NS | NS | NS | |
| Alcohol | ||||||
| Tail abnormilities (%) | Tobacco | NS | NS | NS | NS | |
| Alcohol | ||||||
| Cytoplasmic droplet (%) | Tobacoo | NS | NS | NS | NS | |
| Alcohol | ||||||
| Multiple anomalies index | Tobacoo | r= 0.259 p<0.05 | NS | NS | NS | |
| Alcohol | r= 0.253 p=0.05 | |||||
Statistical analysis was performed using the Spearman Rank Order correlation test. Significant difference with control group (P<0.05). Highly significant difference with control group (P<0.01) NS Not significant
Table 5: Correlation between Sperm parameters and oxidative biomarkers.
Inter-correlation Between the different studied OS BiomarkersThe inter-correlation between the antioxidant enzymes activities and the NBT percentage is detailed in Table 5. Interestingly, there was a significant positive correlation between the high amounts of NBT and the decreased concentrations of seminal CAT, and GPx (p<0.001). In addition, positive and highly [24] significant correlations were found between the different antioxidant enzymes activities (p<0.01).
Multivariable regression analysisMultivariable regression analysis [25] revealed that the OS biomarkers especially SOD activity (z-score=0.484; p=0.002) were associated more with tobacco consumption than alcohol abuse (p>0.05) Table 6 and Table 7.
| Seminal OS biomarkers | ||||
|---|---|---|---|---|
| NBT | SOD | GPx | Catalase | |
| 1 | - | - | - | - |
| 2 | NS | - | - | |
| 3 | NS | r= 0.385 | - | - |
| p<0.01 | ||||
| 4 | r= -0.397 | r= 0.414 | NS | - |
| p<0.01 | p<0.01 | |||
Statistical analysis was performed using the Spearman Rank Order correlation test.
*Significant difference with control group (P<0.05). **Highly significant difference with control group (P<0.01). NS=Not significant
Table 6: Inter-correlation between the seminal OS [26] biomarkers Seminal OS biomarkers
|
B |
SE |
β |
t |
p |
|
|---|---|---|---|---|---|---|
NBT (%) |
Tobacco |
-0.01 |
0.01 |
-0.16 |
1.06 |
0.3 |
Alcohol |
-0 |
0.01 |
-0.1 |
0.6 |
0.55 |
|
SOD (U/ mg P) |
Tobacco |
0.288 |
0.9 |
0.484 |
3.22 |
0 |
Alcohol |
0.056 |
0.07 |
0.127 |
0.76 |
0.45 |
|
Gpx (mmol oxide GSH/min/mg P) |
Tobacoo |
0 |
0 |
-0.1 |
0.61 |
0.54 |
Alcohol |
0 |
0 |
-0.02 |
0.12 |
0.9 |
|
CAT (mmol H2O2/min/ mg P) |
Tobacco |
0 |
0 |
-0.18 |
1.32 |
0.2 |
Alcohol |
0 |
0 |
-0.15 |
1.02 |
0.31 |
B unstandardized regression coefficient, SE standard error for the unstandardized regression coefficient, β standardized regression coefficient, t t-test statistic, p probability value.
Table 7: Multivariate regression analysis to assess the independent association between Tobacco consumption and Alcohol abuse with the seminal OS biomarkers.
Discussion
In the current study, an attempt was made to assess the effects of smoking and alcohol consumption on sperm morphological characteristics as well as its relation with OS biomarkers that included superoxide anion (O2), Superoxide Dismutase (SOD), Catalase (CAT) Glutathione Peroxidase (GPx). This was aimed at finding a correlation between sperm quality and oxidative damage level of smoking and drinking teratozoospermic men [27-32].
The correlation between sperm morphological abnormalities and smoking/alcohol has been little emphasized. Interestingly, in this report, we found a significant increase in special morphological abnormalities such as midpiece abnormalities, cytoplasmic excess, irregular head, microcephalic head, and double head, associated with smoking in men with isolated teratozoospermia. In this line, some case-control studies have demonstrated that smoking is associated with a lower sperm quality/function and increased morphological defects, which is partly in accordance with our findings. Similarly, another study carried out on 140 subjects showed that smoking patients are at higher risk of developing teratozoospermia [33-35].
Additionally, we showed that spermatozoon abnormal morphological defects, particularly head defects, were correlated with tobacco consumption, which might be attributed to an increased OS rate and impaired antioxidant capacity in the seminal plasma of infertile men. The percentage of spermatozoa producing O2. was higher and the activities of the studied antioxidant enzymes were lower in teratozoospermic smokers as compared to teratozoospermic non-smokers. However, we did not reach statistical significance, which is probably due to the limited number of the studied cases. This is supported by the findings of the previous research which indicated that smoking in a group of idiopathic infertile men was significantly associated with the percentages of sperm with abnormal morphology and round head, higher level of DNA fragmentation, higher lipid peroxidation and lower antioxidant enzymes. Nevertheless, a handful of studies have shown that smoking detrimentally affects seminal and blood biochemical parameters by inducing ROS overproduction and a concomitant decrease in antioxidant activities [36].
The particularly destructive aspect of smoking on human spermatozoa may be attributed to its major metabolite cotinine and trans-3 hydroxycotinine. Such toxic components detected in the seminal plasma of smokers prove that the composition of tobacco crosses the blood-testis barrier and creates a toxic environment for the spermatozoa. When present in the epididymis, nicotine and cotinine of tobacco disturb its normal functioning, [37-45] particularly alpha-1,4 glycosidase enzyme activity. This inhibits spermatozoa secondary maturation contributing to teratozoospermia. Besides, it promotes ROS overproduction leading to an OS that causes sperm DNA damage, apoptosis and contributes to teratozoospermia.
The present study has also shown that alcohol has a significant negative effect on semen parameters. Teratozoospermic drinkers showed an increased percentage of spermatozoa with midpiece abnormalities and cytoplasmic excess, head defects such asmicrocephalic, macrocephalic, and irregular heads, and tail defects such as bent tail. The present study is the first to report a strong relationship between total percentage of atypical sperm forms, and detailed sperm morphological characteristics and alcohol consumption in patients with isolated teratozoospermia. Regarding alcohol abuse, it has been reported that it produces progressive damage to sperm morphology and spermatogenesis. In a study of 100 patients, expert showed a strong link between alcohol consumption and sperm morphology, suggesting that that 73% of chronic alcoholics were teratozoospermic men (p=0.0009). Moreover, expert evaluated the reproductive function in 1300 chronic alcoholics and 300 non-alcoholic volunteers. They found that the average rate of abnormal forms, head sperm abnormalities, and tail sperm abnormalities in sperm samples of chronic alcoholics increased significantly when compared to the control group.
The effect of alcohol on semen morphology may be mainly [46] due to disturbed endocrine mechanisms as there is a need for the controlled testicular environment and hypothalamic-pituitary-testicular axis for spermatogenesis. In fact, by affecting the cell Sertoli functions, alcohol produces damage to some proteins required for sperm cell production that the Sertoli cells provide. Consequently, alcohol decreases the levels of testosterone, FSH, and LH, which impairs normal spermatozoa morphological development and maturation.
Despite spermatogenesis distributions, several studies have shown that alcohol consumption can cause OS generation by increasing ROS production and interfering with the antioxidant defence mechanism. In our work, ROS production was higher in the group of drinking patients with teratozoospermia as compared to non-drinking patients, but this difference was not significant. Beside, by using powered statistical tests we found that the level of atypical sperm forms as well as the index of morphological abnormalities were associated with ROS overproduction in the drinking group. Regarding GPx and catalase activities, they were higher in the non-drinking group in comparison with consumers but these differences were not significant. These results can be explained by the fact that our study included a limited number of social drinker patients, which may be the reason that we were not able to detect significant differences. Our results contradict the finding of another study that enrolled 34 infertile alcohol drinkers [47]. In that study, a significant difference was found in OS biomarkers such as malondialdehyde and Azote monoxide between infertile drinkers and non-drinkers. On the other hand, alcohol was found to have a positive effect on sperm function and quality, supporting that the opposite effect might be exerted by alcohol on different semen parameters and according to the amount consumed. Besides, an interesting study on Sprague-Dawley rats aimed to distinguish the path of action by which alcohol reduces semen parameters, found that acute and chronic administration of alcohol depleted the levels of testosterone, increased OS and deteriorated spermatic parameters. Thus, it appears that alcohol affects male fertility by impairing hormone secretion and spermatogenesis, which could be due to OS potentiated by the alcohol leading to an increase in abnormal sperm forms.
It can be concluded that one of the reasons behind teratozoospermia is OS, which has become a real concern in recent times. In this respect, we clearly showed that decreased antioxidant enzymes and elevated seminal concentrations were strongly associated with increased levels of morphologically abnormal spermatozoa. It is possible that because of increased OS in the teratozoospermic men, their antioxidant system is not as efficient as that of the fertile men, which could be the reason why morphologically abnormal spermatozoa are more susceptible to cigarette smoke or alcohol. To our knowledge, this work is the first to report the combined effect of smoking and alcohol consumption in significantly impairing sperm morphology. Otherwise, the multivariable regression analysis revealed that the OS [48] biomarkers especially SOD activity was associated more with tobacco consumption than alcohol abuse. The impaired SOD activity in the presence cigarette is due to the accumulation of Superoxide (O2), Hydrogen Peroxide (H2O2), or the products of its decomposition. Therefore, decreased SOD activity is correlated to increased susceptibility of the spermatogenic cells to ROS. OS might be the reason for the correlation of SOD and smoking with sperm morphological defects. Hence, it is clear that spermatozoa will come under OS attack when smoking and alcohol are involving [49].
Conclusion
In summary, the present study suggests that tobacco and alcohol consumption have deleterious effects on semen morphological quality as well as the seminal oxidative balance of infertile men with isolated teratozoospermia. The percentage of sperm ROS production was higher and the activities of antioxidant enzymes were reduced among the smoking/drinking subjects with teratozoospermia, though the changes were not significant. This investigation definitely resolved some controversies regarding the effects of smoking and alcohol consumption on sperm morphological characteristics in infertile men. In accordance with previous research, we support the idea of the multifactorial aetiologies for the pathogenesis of teratozoospermia. Smoking/drinking males who wish to procreate should be specifically warned of these matters.
References
- Awartani KA, Almugbel M, Coskun S (2018) Tertozoospermia and recurrent pregnancy loss. Glob J reprod Med 4: 50-52. [Crossref][Google Scholar]
- Shu JH, Zhang B, Feng GX, Gan XY, Zhou H, et al. (2010) Influence of sperm morphology on the outcomes and neonatal status in IVF-ET. Zhonghua Nan Ke Xue 16: 897-900. [Google Scholar][Pubmed]
- Junca AM, Cohen Bacrie P, Belloc S, Dumont M, Menezo Y, et al. (2009) La teratozoospermie l’heure de l’intracytoplasmic morphologically selected sperm injection (IMSI). Gynecol Obstet Fertil 37: 552-557. [Crossref][Google Scholar][Pubmed]
- Mortimer D, Menkveld R (2001) Sperm Morphology Assessment-Historical Perspectives and Current Opinions Andrology Lab Corner. Reprod Biol 22: 192-205 [Crossref][Google Scholar][Pubmed]
- Brahem S, Mehdi M, Elghezal H, Saad A (2011) Detection of DNA fragmentation and meiotic segregation in human with isolated teratozoospermia. J Assist Reprod Genet 28(1):41-48. [Crossref][Google Scholar][Pubmed]
- Mehdi M, Gmidene A, Brahem S, Guerin JF, Elghezal H, et al. (2012) Aneuploidy rate in spermatozoa of selected men with severe teratozoospermia. Andrologia 1: 139-143. [Crossref][Google Scholar][Pubmed]
- Ammar O, Ajina T, Haouas Z, Sallem A, Zidi I, et al. (2018) Investigation on the origin of sperm morphological defects: oxidative attacks, chromatin immaturity, and DNA fragmentation. Environ Sci Pollut Res 25: 13775-13786. [Crossref][Google Scholar][Pubmed]
- Ammar O, Haouas Z, Hamouda B, Hamdi H, Hellara I, et al. (2019) Relationship between sperm DNA damage with sperm parameters, oxidative markers in teratozoospermic men. Eur J Obstet Gynecol Reprod Biol 233:70-75. [Crossref][Google Scholar][Pubmed]
- Ammar O, Mehdi M, Muratori M (2020) Teratozoospermia: its association with sperm DNA defects, apoptotic alterations and oxidative stress. Andrology 2020;(10):1-12. [Crossref][Google Scholar][Pubmed]
- Ammar O, Tekeya O, Hannachi I, Sallem A, Haouas Z, et al. (2020) Increased Sperm DNA Fragmentation in Infertile Men with Varicocele: Relationship with Apoptosis, Seminal Oxidative Stress, and Spermatic Parameters. Reprod Sci 28: 909-919. [Crossref][Google Scholar][Pubmed]
- La Vignera S, Condorelli RA, Balercia G, Vicari E, Calogero AE, et al. (2013) Does alcohol have any effect on male reproductive function? A review of literature. Asian J Androl 2013;15:221-5. [Crossref][Google Scholar][Pubmed]
- Harlev A, Agarwal A, Gunes SO, Shetty A, du Plessis SS, et al. (2015) Smoking and male infertility: An evidence-based review. World J Mens Health. 33: 143-160 [Crossref][Google Scholar][Pubmed]
- Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer Lindgren L, et al. (2014) Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA -J Am Med Assoc 311: 183-192. [Crossref][Google Scholar][Pubmed]
- Sharma R, Harlev A, Agarwal A, Esteves SC (2010) Cigarette Smoking and Semen Quality: A New Meta-analysis Examining the Effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Euro Urol 70:635-645. [Crossref][Google Scholar][Pubmed]
- Davar R, Sekhavat L, Naserzadeh N (2012) Semen parameters of non-infertile smoker and non-smoker men. J Med Life 15:465-468.[Crossref] [Google Scholar][Pubmed]
- Caserta D, Bordi G, Di Segni N, D Ambrosio A, Mallozzi M, et al. (2013) The influence of cigarette smoking on a population of infertile men and women. Arch Gynecol Obstet 287:813-818. [Crossref][Google Scholar][Pubmed]
- Saleh RA, Agarwal A, Kandirali E, Sharma RK, Thomas AJ, et al. (2002) Leukocytospermia is associated with increased reactive oxygen species production by human spermatozoa. Fertil Steril 78: 1215-1224. [Crossref][Google Scholar][Pubmed]
- Bisht S, Faiq M, Tolahunase M, Dada R (2017) Oxidative stress and male infertility. Nat Rev Uro 14: 470-485. [Crossref][Google Scholar][Pubmed]
- Taha EA, Ez Aldin AM, Sayed SK, Ghandour NM, Mostafa T, et al. (2012) Effect of smoking on sperm vitality, DNA integrity, seminal oxidative stress, zinc in fertile men. Uro 80: 822-825. [Crossref][Google Scholar][Pubmed]
- Calogero A, Polosa R, Perdichizzi A, Guarino F, La Vignera S, et al. (2009) Cigarette smoke extract immobilizes human spermatozoa and induces sperm apoptosis. Reprod Biomed Online 19:564-571. [Crossref][Google Scholar][Pubmed]
- Aboulmaouahib S, Madkour A, Kaarouch I, Sefrioui O, Saadani B, et al. (2018) Impact of alcohol and cigarette smoking consumption in male fertility potential: Looks at lipid peroxidation, enzymatic antioxidant activities and sperm DNA damage. Andrologia 50:3. [Crossref][Google Scholar][Pubmed]
- Ramgir SS, Abilash VG (2019) Impact of smoking and alcohol consumption on oxidative status in Male infertility and sperm quality. Indian J Pharm Sci 81:933-945. [Crossref][Google Scholar]
- Jin Chun Lu, Yu-Feng Huang, Nian Qing Lu (2010) World Health Organization Laboratory Manual for the Examination and Processing of Human Semen. WHO Press 2010. [Google Scholar][Pubmed]
- Auger JEF (2000) Standardisation de la classification morphologique des spermatozoïdes humains selon la methode de David modifiee. Andrologie 358-373. [Crossref][Google Scholar]
- Bailey SM, Landar A, Darley Usmar V (2005) Mitochondrial proteomics in free radical research. Free Rad Bio Med 38:175-188. [Crossref][Google Scholar][Pubmed]
- Flohe LGW (1984) Assays of glutathione peroxidase. Methods Enzym114-121. [Crossref][Google Scholar][Pubmed]
- Beyer WF, Fridovich I (1987) Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal Biochem161:559-566. [Crossref][Google Scholar][Pubmed]
- Tunc O, Thompson J, Tremellen K (2010) Development of the NBT assay as a marker of sperm oxidative stress. Int J Androl 33:13-21. [Crossref][Google Scholar][Pubmed]
- Kunzle R, Mueller MD, Hanggi W, Birkhauser MH, Drescher H, et al.(2003) Semen quality of male smokers and nonsmokers in infertile couples. Fertil Steril 79:287-291.[Crossref][Google Scholar][Pubmed]
- Asare Anane H, Bannison SB, Ofori EK, Ateko RO, Bawah AT, et al. (2016) Tobacco smoking is associated with decreased semen quality. Reprod Health 13:90. [Crossref][Google Scholar][Pubmed]
- Elshal MF, El Sayed IH, Elsaied MA, El Masry SA, Kumosani TA, et al. (2019) Sperm head defects and disturbances in spermatozoal chromatin and DNA integrities in idiopathic infertile subjects: Association with cigarette smoking. Clin Biochem 42:589-594. [Crossref][Google Scholar][Pubmed]
- Mostafa T, Tawadrous G, Roaia MMF, Amer MK, Kader RA, et al. (2006) Effect of smoking on seminal plasma ascorbic acid in infertile and fertile males. Andrologia 38:221-224. [Crossref][Google Scholar][Pubmed]
- Dai JB, Wang ZX, Qiao ZD (2015) The hazardous effects of tobacco smoking on male fertility. Asian J Andro 17: 954-960. [Crossref][Google Scholar][Pubmed]
- Chari MG, Colagar AH (2011) Seminal plasma lipid peroxidation, total antioxidant capacity, and cigarette smoking in asthenoteratospermic men. J Mens health 8:43-49. [Crossref][Google Scholar]
- Ernster VL, Grady DG, Greene JC, Walsh M, Robertson P, et al. (1990) Smokeless Tobacco Use and Health Effects Among Baseball Players. JAMA J Am Med Assoc 264:218-224.[Crossref][Google Scholar][Pubmed]
- Sunanda P, Panda B, Dash C, Ray PK, Padhy RN, et al. (2014) Prevalence of abnormal spermatozoa in tobacco chewing sub-fertile males. J Hum Reprod Sci 7:136-142. [Crossref][Google Scholar][Pubmed]
- Said TM, Ranga G, Agarwal A (2005) Relationship between semen quality and tobacco chewing in men undergoing infertility evaluation. Fertil Steril 84 :649-653. [Crossref][Google Scholar][Pubmed]
- Verma MK. (2019) Association of lifestyle factors like alcohol and tobacco consumption with semen abnormalities. J Med Sci Clin Res 7:695-700. [Crossref][Google Scholar][Pubmed]
- Saud HK (2014) Chromatin integrity in human ejaculate sperm of smokers and non-smokers patients and its relationship to seminal oxidative stress parameters. Int J Biosci 2014;(9):126-141. [Crossref][Google Scholar][Pubmed]
- Marinelli D, Gaspari L, Pedotti P, Taioli E. (2004) Mini review of studies on the effect of smoking and drinking habits on semen parameters. Int J Hyg Environ Health 207: 185-192. [Crossref][Google Scholar][Pubmed]
- Ricci E, Al Beitawi S, Cipriani S, Candiani M, Chiaffarino F, et al. (2017) Semen quality and alcohol intake: A systematic review and meta-analysis. Reprod Biomed Online 34: 38-47. [Crossref][Google Scholar][Pubmed]
- Boeri L, Capogrosso P, Ventimiglia E, Pederzoli F, Cazzaniga W, et al. (2019) Heavy cigarette smoking and alcohol consumption are associated with impaired sperm parameters in primary infertile men. Asian J Androl 2: 478-485. [Crossref][Google Scholar][Pubmed]
- Gaur DS, Talekar MS, Pathak VP (2010) Alcohol intake and cigarette smoking: Impact of two major lifestyle factors on male fertility. Indian J Pathol Microbiol 53:35-40. [Crossref][Google Scholar][Pubmed]
- Muthusami KR, Chinnaswamy P (2005) Effect of chronic alcoholism on male fertility hormones and semen quality. Fertil Steril 84:919-924. [Crossref][Google Scholar][Pubmed]
- Maneesh M, Dutta S, Chakrabarti A, Vasudevan DM (2006) Alcohol abuse-duration dependent decrease in plasma testosterone and antioxidants in males. Indian J Physiol Pharmacol 50:291. [Google Scholar][Pubmed]
- Oremosu AA, Akang EN (2015) Impact of alcohol on male reproductive hormones, oxidative stress and semen parameters in Sprague-Dawley rats. Middle East Fertil Soc J 20(2):114–118. [Crossref][Google Scholar][Pubmed]
- Ammar O, Mehdi M, Tekeya O, Neffati F, Haouas Z, et al. (2019) Novel association between apoptotic sperm biomarkers with seminal biochemical parameters and acetylcholinesterase activity in patients with teratozoospermia. J Assist Reprod Genet 36. [Crossref][Google Scholar][Pubmed]
- Belcheva A, Ivanova Kicheva M, Tzvetkova P, Marinov M (2004) Effects of cigarette smoking on sperm plasma membrane integrity and DNA fragmentation. Int J Androl 27:296-300. [Crossref][Google Scholar][Pubmed]
- Agarwal A, Tvrda E, Sharma R (2014) Relationship amongst teratozoospermia, seminal oxidative stress and male infertility. Reprod Biol Endocrinol 12:2-9. [Crossref][Google Scholar][Pubmed]