Case Report, J Genet Disor Genet Rep Vol: 12 Issue: 5
Penile Sebaceous Adenoma with Sporadic Loss of MLH-1 and PMS2 Protein Expression: A Case Report and Review of Literature
Blaire A. Anderson1,2*, Shaofeng Yan1 and Bing Ren1
1Department of Pathology and Laboratory Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, United States of America
2Department of Medicine, Dartmouth College, Hanover, United States of America
*Corresponding Author: Blaire A. Anderson,
Department of Pathology and
Laboratory Medicine, Dartmouth-Hitchcock Medical Center, Lebanon, United
States of America, Department of Medicine, Dartmouth College, Hanover, United
States of America
E-mail: blaire.anderson.med@dartmouth.edu
Received date: 25 September, 2023, Manuscript No. JGDGR-23-114660;
Editor assigned date: 28 September, 2023, PreQC No. JGDGR-23-114660 (PQ);
Reviewed date: 11 October, 2023, QC No JGDGR-23-114660;
Revised date: 18 October, 2023, Manuscript No JGDGR-23-114660 (R);
Published date: 25 October, 2023, DOI: 10.4172/2576-1439.1000224
Citation: Anderson AB, Yan S, Ren B (2023) Penile Sebaceous Adenoma with Sporadic Loss of MLH-1 and PMS2 Protein Expression: A Case Report and Review of Literature. J Genet Disor Genet Rep 12:5.
Abstract
Sebaceous adenoma is a rare cutaneous neoplasm. It typically presents in the sixth decade of life and beyond as a yellow or tan-pink papule in sun-exposed areas, particularly the head and neck. Though sebaceous adenoma is widely considered a benign entity, it can be a presenting condition in patients with Muir-Torre Syndrome (MTS), a rare subtype of Hereditary Nonpolyposis Colorectal Cancer (HNPCC), characterized by an increased risk for visceral malignancies and the development of sebaceous neoplasms. HNPCC and MTS result from germline mutations in DNA Mismatch Repair (MMR) protein genes including MLH1, PMS2, MSH2, MSH6, and EPCAM. Cases of sebaceous adenoma arising in non-sun-exposed areas in patients under 60 years of age with personal or family histories suggestive of HNPCC/MTS are routinely evaluated for loss of Deoxyribonucleic Acid (DNA) mismatch repair proteins and possible germline mutations. Here we present a rare case of penile sebaceous adenoma with unusual histologic features and sporadic loss of MLH1 and PMS2 protein expression in a 52-year-old patient.
Keywords: Muir-torre syndrome; Hereditary nonpolyposis
colorectal cancer; Mismatch Repair, Deoxyribonucleic acid;
MLH1; PMS2
Introduction
Sebaceous adenoma is a rare benign neoplasm of sebaceous origin. Clinically, sebaceous adenoma presents as a yellow to tan-pink papule with or without central umbilication and ulceration [1,2]. Sebaceous adenoma is typically a solitary lesion arising in elderly individuals (>60 years) in sun-exposed areas, most commonly the head and neck [1]. Histopathologically sebaceous adenoma presents as a wellcircumscribed, lobulated growth with an expanded population of basaloid cells at the periphery of lobes and mature sebocytes situated centrally [3]. Unusual clinical presentations, including sebaceous adenoma arising in younger patients (>60 years); multiple sebaceous adenomas; and sebaceous adenoma arising in areas other than the head and neck should raise suspicion for a diagnosis of Muir-Torre Syndrome (MTS) [4]. MTS is a rare subset of Hereditary Nonpolyposis Colorectal Cancer (HNPCC) characterized by the development of sebaceous neoplasms in addition to visceral malignancies associated with HNPCC including colorectal and endometrial carcinoma. Rare histologic patterns, including keratoacanthoma-like morphology and predominantly cystic features, can also be seen in the setting of MTS-associated sebaceous adenoma [3-5].
MTS is an autosomal dominant condition caused by germline mutations in DNA mismatch repair genes including MLH1, PMS2, MSH2, MSH6, and EPCAM resulting in Microsatellite Instability (MSI) [5,6]. In patients with MTS, sebaceous neoplasms (including sebaceous adenoma, secaceoma, and sebaceous carcinoma) may present synchronously or metachronously with visceral malignancies [3]. One study (n=205) reported presentation of sebaceous neoplasm prior to the development of a visceral malignancy in 22% of patients with MTS [7]. Other studies have shown that up to 41% of patients with MTS will present with sebaceous neoplasm prior to or concurrent with visceral malignancy [8,9]. Patients diagnosed with MTS and their family members require genetic counseling as well as close surveillance for the development of visceral and cutaneous malignancies. Given the increased risk for malignancy in patients with MTS and availability of effective surveillance and treatment options, Immuno-Histochemical (IHC) analysis of sebaceous neoplasms for loss of MMR protein expression has been suggested as screening method for undiagnosed cases [4]. Whether this screening should be applied universally to all sebaceous neoplasms or in a selective manner based on clinical characteristics and personal or family history of MTS/HNPCC associated malignancy remains a matter of debate [10]. IHC analysis is not capable of distinguishing between loss of MMR protein expression due to sporadic versus germline mutations in MMR genes; consequently, this method of screening for MTS suffers from a high false positive rate with MMR protein loss being detected in the absence of germline mutations in MMR genes [4]. IHC analysis for loss of MLH1, PMS2, MSH2, MSH6 is not used as a diagnostic test for MTS, but results are used to guide decision making and further screening recommendations including genetic testing for germline mutations.
Here we present a rare case of penile sebaceous adenoma with sporadic loss of MLH1 and PMS2 protein expression in a 52-year-old patient. To our knowledge, this is the only case of penile sebaceous adenoma reported in a patient under 60 years of age, and the only case in which IHC and genetic testing are reported.
Case Presentation
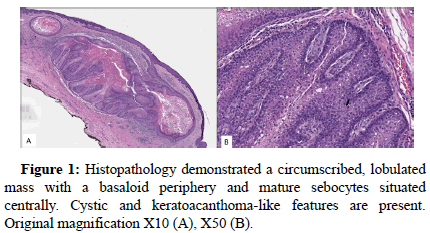
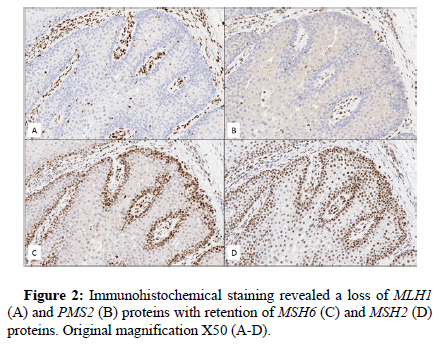
A 52-year-old man with a past medical history of inverted papilloma and sinonasal squamous cell carcinoma presented with an 8 × 6 mm cystic lesion of the dorsal penis. The lesion was excised in an ellipse superficial to the fascia and submitted for pathologic examination. Grossly, the excised specimen was a tan-pink papule with a discrete, central cystic structure. Microscopic exam demonstrated a well-circumscribed, lobulated lesion. Distinct basaloid cells were present at the periphery of the lobes with bland, mature sebocytes located centrally. Cystic and keratoacanthoma-like features were present. A diagnosis of sebaceous adenoma was rendered. Immunohistochemical stains for DNA mismatch repair proteins revealed absence of nuclear staining for MLH1 and PMS2 and intact nuclear staining for MSH2 and MSH6 in lesional cells. Molecular testing for MLH1 promoter methylation was negative, suggesting loss of MLH1 protein expression was likely the result of a somatic or germline mutation in the MLH1 gene (Figures 1 and 2).
The patient was subsequently referred by his primary care provider for genetic counseling and colonoscopy. A review of the patient’s family history revealed pancreatic cancer in the maternal grandmother. Genetic testing with the invitae common hereditary cancers panel, which includes MLH1, MSH2, MSH6, PMS2, and EPCAM, was recommended. The patient initially elected to delay genetic testing in order to discuss the matter with his family and consider all possible risks and benefits, but promptly underwent colonoscopy. The colonoscopy was unremarkable. When the patient subsequently elected to proceed with genetic testing, no germline mutations were detected. This result suggests a sporadic mutation was responsible for the development of our patient’s sebaceous adenoma.
Discussion
Universal immunohistochemical analysis for loss of MMR proteins to screen for HNPCC in patients presenting with colorectal and endometrial carcinoma is a widely accepted practice [11,12]. Use of universal IHC analysis in sebaceous neoplasms to screen for MTS is more controversial.
Arguments against universal screening include financial and ethical objections, as well as possible harm to patients with false negative results. At present, no comprehensive cost-benefit analyses of universal IHC screening in sebaceous neoplasms have been published [13]. Without cost-benefit analyses, it is not possible to evaluate the potential financial harms or benefits universal screening protocols could pose for patients and healthcare institutions. Ethical objections to universal screening include a lack of established protocols for obtaining informed consent from patients prior to preforming IHC analysis. One study in patients diagnosed with HNPCC has shown the majority of patients support an informed consent process prior to IHC screening [14]. Additionally, The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group has recommended obtaining informed consent prior to completing IHC screening on CRC tissue in patients with HNPCC [15]. However, current clinical practices for obtaining informed consent vary with one study reporting obtainment of written consent prior to IHC analysis in only 7.1% of cases [16,17]. Informed consent is an important consideration for patients presenting with sebaceous neoplasms concerning for MTS, particularly given the lower sensitivity and specificity of IHC screening in sebaceous neoplasms compared to colorectal tumors [18,19]. False-positive results are not uncommon in IHC analyses of sebaceous neoplasms with estimates ranging from 56%-71% [4,20]. False-positive IHC results can lead to patient harm. Cases of patients erroneously diagnosed with MTS based on falsepositive IHC results have been reported; these patients were subsequently subjected to unnecessary surveillance procedures including yearly colonoscopies [18]. Indeed, the patient we report herein underwent an unnecessary colonoscopy prior to completing genetic testing.
Several selective screening models, most notably the Mayo MTS score, have been proposed to increase pretest probability of detecting germline mutations and limit unnecessary testing for patients with sebaceous neoplasms [21,22]. These models propose the use of algorithms which incorporate clinical characteristics including patient age, presence of multiple sebaceous neoplasms, and family/personal history of HNPCC-related malignancies to screen patients presenting with sebaceous neoplasms. IHC analysis is recommended only in those patients who present with compelling clinical characteristics in addition to a sebaceous neoplasm. Advocates of the selective screening approach note the importance of evaluating for personal/ family history of HNPCC-associated malignancy when screening for MTS, while recognizing that certain unusual clinical features also warrant investigation [18].
Proponents of universal IHC analysis have advocated for its use based on the difficulty of obtaining accurate family and personal history of HNPCC-associated cancers, the possibility of missing cases of MTS when an age cutoff is applied, and the relative ease of use and rapid turnaround times associated with IHC analysis [23].
Conclusion
In summary, we present a novel case of penile sebaceous adenoma in a patient under 60 years of age with rare histologic features and sporadic loss of MLH1 and PMS2 protein expression. This case highlights the current debate regarding universal versus selective IHC analysis of sebaceous neoplasms and the potential harms to patients with false-positive IHC results. More studies are needed to determine the most appropriate model for screening of sebaceous neoplasms.
Ideal screening recommendations would capture a majority of MTS cases while minimizing cost and potential harm to patients.
References
- Flux K. (2017) Sebaceous Neoplasms. Surg Pathol Clin 10(2):367-382.
[Crossref] [Google scholar] [Pubmed]
- Zaballos P, Gómez MI, Martin JM, Bañuls J (2018) Dermoscopy of Adnexal Tumors. Dermatol Clin. 36(4):397-412.
[Crossref] [Google scholar] [Pubmed]
- Ferreira I, Wiedemeyer K, Demetter P, Adams DJ, Arends MJ, et al. (2020) Update on the pathology, genetics and somatic landscape of sebaceous tumours. Histopathology. 76(5):640-649.
[Crossref] [Google scholar] [Pubmed]
- Roberts ME, Riegert JDL, Thomas BC, Kandelaria MR, Colleen ST, et al. (2014) A clinical scoring system to identify patients with sebaceous neoplasms at risk for the Muir–Torre variant of Lynch syndrome. Genet Med 16(9):711-716.
[Crossref] [Google scholar] [Pubmed]
- Gay JT, Troxell T, Gross GP (2021) Muir-Torre Syndrome. StatPearls.
[Pubmed]
- Lazar AJF, Lyle S, Calonje E (2007) Sebaceous neoplasia and Torre–Muir syndrome. Curr Diagnostic Pathol 13(4):301-319. .
[Crossref] [Google scholar] [Pubmed]
- Akhtar S, Oza KK, Khan SA, Wright J (1999) Muir-Torre syndrome: Case report of a patient with concurrent jejunal and ureteral cancer and a review of the literature. J Am Acad Dermatol 41(5):681-686.
[Crossref] [Google scholar] [Pubmed]
- Cohen PR, Kohn SR, Kurzrock R (1991) Association of sebaceous gland tumors and internal malignancy: The muir-torre syndrome. Am J Med 90(1):606-613.
[Crossref] [Google scholar] [Pubmed]
- Schwartz RA, Torre DP (1995). The Muir-Torre syndrome: A 25-year retrospect. J Am Acad Dermatol 33(1):90-104.
[Crossref] [Google scholar] [Pubmed]
- Vidal CI, Armbrect EA, Andea AA, Angela KB, Nneka IC, et al. (2019)Appropriate use criteria in dermatopathology: Initial recommendations from the American Society of Dermatopathology. J Am Acad Dermatol 80(1):189-207.
[Crossref] [Google scholar] [Pubmed]
- Adar T, Rodgers LH, Shannon KM, Makoto YMD, Tianle MMD, et al. (2018) Universal screening of both endometrial and colon cancers increases the detection of Lynch syndrome. Cancer 124(15):3145-3153.
[Crossref] [Google scholar] [Pubmed]
- Menahem B, Alves A, Regimbeau JM, Sabbagh C (2019) Lynch Syndrome: Current management In 2019. J Visc Surg 156(6):507-514.
[Crossref] [Google scholar] [Pubmed]
- Kim RH, Nagler AR, Meehan SA (2016) Universal immunohistochemical screening of sebaceous neoplasms for Muir-Torre syndrome: Putting the cart before the horse? J Am Acad Dermatol 75(5):1078-1079.
- Subramonian A, Smith D, Dicks E, Dawson L, Borgaonkar M, et al. (2020) Universal tumor screening for lynch syndrome: Perspectives of patients regarding willingness and informed consent. Per Med 17(5):373-387.
[Crossref] [Google scholar] [Pubmed]
- Berg AO, Armstrong K, Botkin J, Calonge N, Haddow J, et al. (2009) Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med 11(1):35-41.
[Crossref] [Google scholar] [Pubmed]
- Cohen SA (2014) Current Lynch Syndrome Tumor Screening Practices: A Survey of Genetic Counselors. J Genet Couns 23(1):38-47.
[Crossref] [Google scholar] [Pubmed]
- Gaff CL, Rogers MT, Frayling IM. (2007) Genetic counselling and consent for tumour testing in HNPCC. Clin Genet 71(5):400-405.
[Crossref] [Google scholar] [Pubmed]
- Roberts ME, Riegert JDL, Thomas BC, Colleen ST, Michael GH, et al. (2013) Screening for Muir-Torre syndrome using mismatch repair protein immunohistochemistry of sebaceous neoplasms. J Genet Couns 22(3):393-405.
[Crossref] [Google scholar] [Pubmed]
- Everett JN, Raymond VM, Dandapani M ,Monica M, Wendy K, et al. (2014) Screening for germline mismatch repair mutations following diagnosis of sebaceous neoplasm. JAMA dermatology 150(12):1315-1321.
[Crossref] [Google scholar] [Pubmed]
- Joly MO, Attignon V, Saurin JC, Françoise D, Dominique L, et al. (2015) Somatic MMR Gene Mutations as a Cause for MSI-H Sebaceous Neoplasms in Muir–Torre Syndrome-Like Patients. Hum Mutat 36(3):292-295.
[Crossref] [Google scholar] [Pubmed]
- John AM, Schwartz RA. (2016) Muir-Torre syndrome (MTS): An update and approach to diagnosis and management. J Am Acad Dermatol 74(3):558-566.
[Crossref] [Google scholar] [Pubmed]
- Abbas O, Mahalingam M (2009) Cutaneous sebaceous neoplasms as markers of Muir-Torre syndrome: a diagnostic algorithm. J Cutan Pathol 36(6):613-619.
[Crossref] [Google scholar] [Pubmed]
- Jessup CJ, Redston M, Tilton E, Reimann JDR (2016) Importance of universal mismatch repair protein immunohistochemistry in patients with sebaceous neoplasia as an initial screening tool for Muir-Torre syndrome. Hum Pathol 49:1-9.
[Crossref] [Google scholar] [Pubmed]